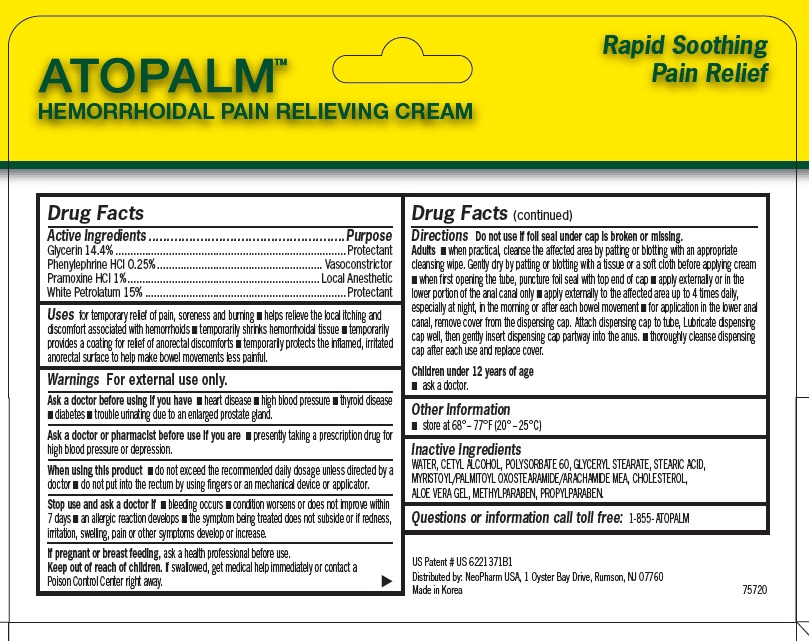
Uses
for temporary relief of pain, soreness and burning ■ helps relieve the local itching and discomfort associated with hemorrhoids ■ temporarily shrinks hemorrhoidal tissue ■ temporarily provides a coating for relief of anorectal discomforts ■ temporarily protects the inflamed, irritated anorectal surface to help make bowel movements less painful.
Warnings
For external use only.
Ask a doctor before using if you have
■ heart disease ■ high blood pressure ■ thyroid disease ■ diabetes ■ trouble urinating due to an enlarged prostate gland
Ask a doctor of pharmacist before use if you are
■ presently taking a prescription drug for high blood pressure or depression.
When using this product
■ do not exceed the recommended daily dosage unless directed by a doctor ■ do not put into the rectum by using fingers or an mechanical device or applicator.
Directions
Do not use if foil seal under cap is broken or missing.
Adults ■ when practical, cleanse the affected area by patting or blotting with an appropriate cleansing wipe. Gently dry by patting or blotting with a tissue or a soft cloth before applying cream ■ when first opening the tube, puncture foil seal with top end of cap ■ apply externally or in the lower portion of the anal canal only ■ apply externally to the affected area up to 4 times daily, especially at night, in the morning or after each bowel movement ■ for application in the lower anal canal, remove cover from the dispensing cap. Attach dispensing cap to tube, Lubricate dispensing
cap well, then gently insert dispensing cap partway into the anus. ■ thoroughly cleanse dispensing cap after each use and replace cover.
Children under 12 years of age
■ ask a doctor.
Inactive Ingredients
WATER, CETYL ALCOHOL, POLYSORBATE 60, GLYCERYL STEARATE, STEARIC ACID, MYRISTOYL/PALMITOYL OXOSTEARAMIDE/ARACHAMIDE MEA, CHOLESTEROL, ALOE VERA GEL, METHYLPARABEN, PROPYLPARABEN.
Product Labeling
Rapid Soothing Pain Relief
US Patented Technology
ATOPALM
HEMORRHOIDAL PAIN RELIEVING CREAM
With US Patented MLE Technology
Rapid Relief from Painful Burning, Itching, External Discomfort, Shrinks Swollen Hemorrhoidal Tissue
Soothing cream with pain relieving ingredient. Temporarily Protects Irritated Tissue, Rapid Relief from External Discomfort
Strong Pain Relief – Soothes Irritated Hemorrhoidal Tissue
Net Wt. 1 oz /28.3 g
US Patent # US 6221371B1
Distributed by: NeoPharm USA, 1 Oyster Bay Drive, Rumson, NJ 07760
Made in Korea