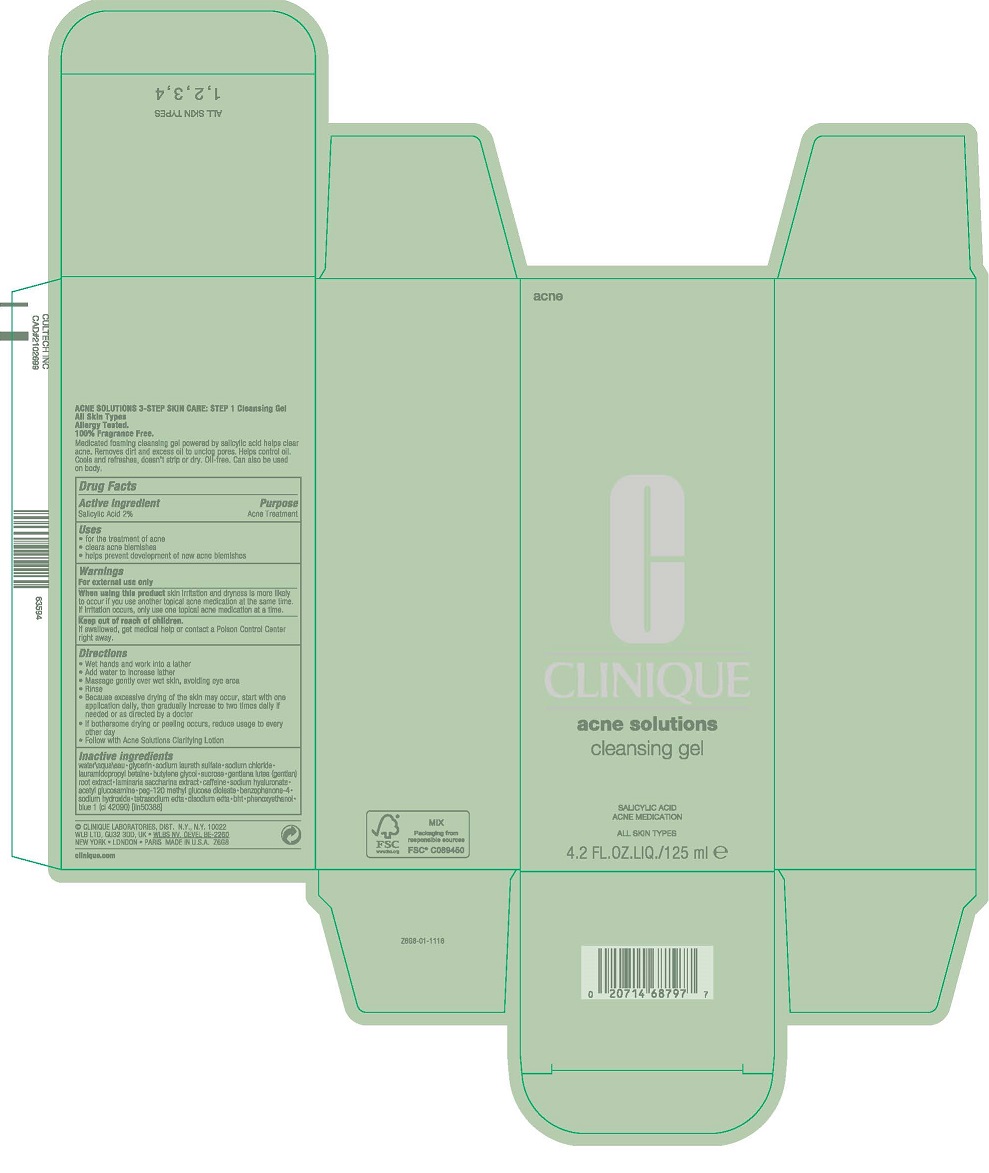
Warnings
For external use only
Directions
- Wet hands and work into a lather
- Add water to increase lather
- Massage gently over wet skin, avoiding eye area
- Rinse
- Use A.M. and P.M.
- If bothersome drying or peeling occurs, reduce usage to every other day
- Follow with Acne Solutions Clarifying Lotion
Inactive ingredients
water\aqua\eau [] glycerin [] sodium laureth sulfate [] sodium chloride [] lauramidopropyl betaine [] butylene glycol [] sucrose [] gentiana lutea (gentian) root extract [] laminaria saccharina extract [] caffeine [] sodium hyaluronate [] acetyl glucosamine [] peg-120 methyl glucose dioleate [] benzophenone-4 [] sodium hydroxide [] tetrasodium edta [] disodium edta [] bht [] phenoxyethanol [] blue 1 (ci 42090) <iln50388>