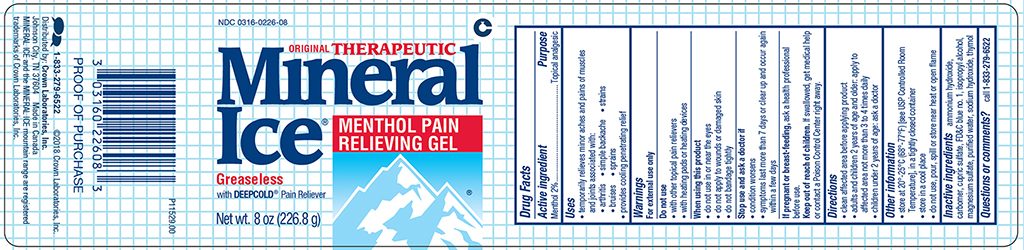
Active ingredient
Menthol 2%
Purpose
Topical analgesic
Uses
● temporarily relieves minor aches and pains of muscles and joints associated with:
● arthritis ● simple backache ● strains
● provides cooling penetrating relief
Warnings
For external use only
Do not use
- with other topical pain relievers
- with heating pads or heating devices
When using this product
- do not use in or near the eyes
- do not apply to wounds or damaged skin
- do not bandage tightly
Stop use and ask a doctor if
- condition worsens
- symptoms last more than 7 days or clear up and occur again within a few days
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- clean affected area before applying product
- adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
Other information
- store at 20
o - 25
oC (68
o - 77
oF) [see USP Controlled Room Temperature], in a tightly closed container
- store in a cool place
- do not use, pour, spill or store near heat or open flame
Inactive ingredients
ammonium hydroxide, carbomer, cupric sulfate, FD&C blue no. 1, isopropyl alcohol, magnesium sulfate, purified water, sodium hydroxide, thymol
Questions or comments?
call
1-833-279-6522
Additional Information Listed on Other Panels
1-833-279-6522 ©Crown Laboratories, Inc.
Distributed by:
Crown Laboratories, Inc.
Johnson City, TN 37604 ©2018 Made in Canada.
Mineral Ice and the Mineral Ice mountain range are registered trademarks of Crown Laboratories, Inc.
Principal Display
NDC 0316-0226-08
ORIGINAL THERAPEUTIC
Mineral Ice®
Menthol Pain Relieving Gel
Greaseless with DEEPCOLD® Pain Reliever
Net wt. 8 oz (226.8 g)
P11529.00