ALCOHOL- alcohol spray
ALCOHOL- alcohol gel
Hello Bello
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
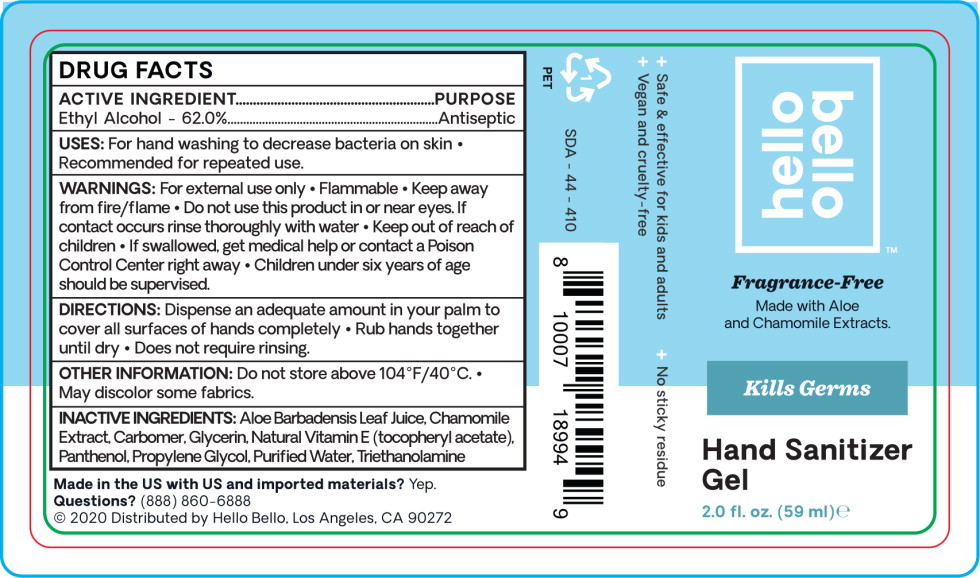
ACTIVE INGREDIENT
Ethyl Alcohol - 62.0%
USES:
For hand washing to decrease bacteria on skin
- Recommended for repeated use.
WARNINGS:
For external use only
- Flammable
- Keep away from fire/flame
- Do not use this product in or near eyes. If contact occurs rinse thoroughly with water
- Keep out of reach of children
- If swallowed, get medical help or contact a Poison Control Center right away
- Children under six years of age should be supervised.
DIRECTIONS:
Dispense an adequate amount in your palm to cover all surfaces of hands completely
- Rub hands together until dry
- Does not require rinsing.
OTHER INFORMATION:
Do not store above 104°F/40°C.
- May discolor some fabrics.
INACTIVE INGREDIENTS:
Aloe Barbadensis Leaf Juice. Chamomile Extract Carbomer, Glycerin, Natural Vitamin E (tocopheryl acetate), Panthenol, Propylene Glycol, Purified Water, Triethanolamine
Principal Display Panel – 118 mL Bottle Label
Non-sterile Solution
hello
bello™
Alcohol Antiseptic 80%
Topical Solution
80% Alcohol
Hand Sanitizer
Spray
4 fl. oz. (118 ml) e
Principal Display Panel – 354 mL Bottle Label
hello
bello™
Fragrance-Free
Kills Germs
Hand Sanitizer
Gel
12 fl. oz. (354 ml) e
Principal Display Panel – 59 mL Bottle Label
hello
bello™
Fragrance-Free
Made with Aloe
and Chamomile Extracts.
Kills Germs
Hand Sanitizer
Gel
2.0 fl. oz. (59 ml) e