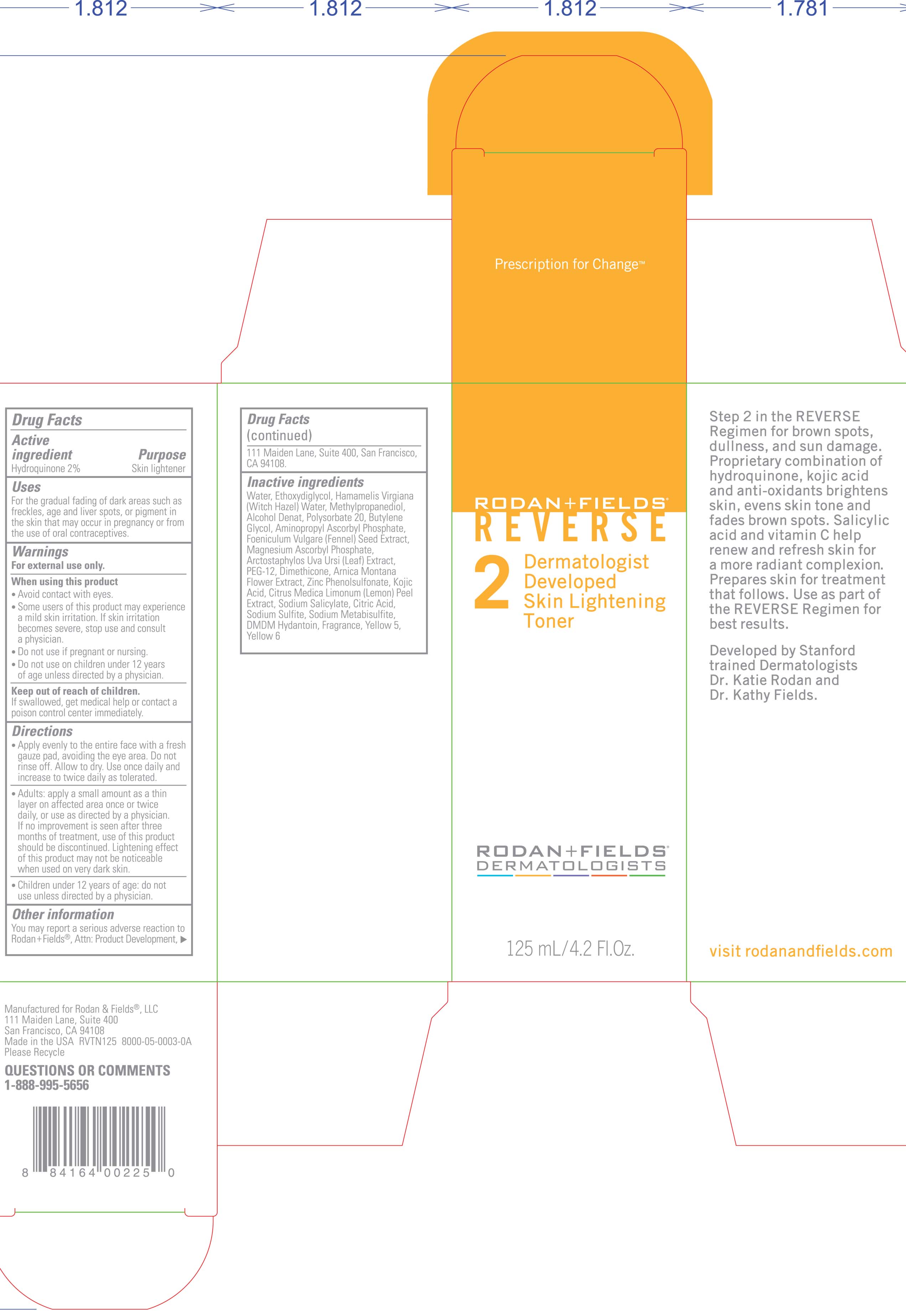
Hydroquinone 2%
Keep out of reach of children
if swallowed, get medical help or contact a poison control center immediately.
avoid contact with eyes.
some
user of this product may experience a mild skin irritation. if skin
irritation becomes severe , stop use and consult a physician.
do not use if pregnant or nursing.
do not use on children under 12 years of age unless directed by a physician.apply evenly to the entire face with a fresh gauze pad, avoiding the
eye area. do not rinse off, allow to dry. use once daily and increase
to twice daily as tolerated.
Adult: apply a small amount as a
think layer in affected area once or twice daily, or use as directed by
a physician . if no improvement is seen after three months of
treatment, use of this product should be discontinued . lightening
effect of this product may not be noticeable when used on very dark
skin.
children under 12 years of age: do not use unless directed by a physician.
Water (aqua), Ethoxydiglycol, Hamamelis Virgiana ( Witch Hazel) Water, Methylpropanediol, Alcohol Denat, Polysorbate 20, Butylene Glycol, Aminopropyl Ascorbyl Phosphate, Foeniculum Vulgare ( Fennel) Seed Extract, Magnesium Ascorbyl Phosphate, Arctostaphylos Uva Ursi (leaf) Extract, PEG-12, Dimethicone, Arnica Montana Flower Extract, Zinc Phenolsulfonate, Kojic Acid, Citrus Medica Limonum ( Lemon) Peel Extract, Sodium Salicylate, Citric Acid, Sodium Sulfite, Sodium Metabisulfite, DMDM Hydantoin, Fragrance, Yellow 5, Yellow 6