BOXED WARNING
Cardiovascular Risk
- NSAIDs may cause an increased risk of serious cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal. This risk may increase with duration of use. Patients with cardiovascular disease or risk factors for cardiovascular disease may be at greater risk. (See WARNINGS)
- SULINDAC is contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery (See WARNINGS).
Gastrointestinal Risk
- NSAIDs cause an increased risk of serious gastrointestinal adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal events. (See WARNINGS)
DESCRIPTION
Sulindac is a non-steroidal, anti-inflammatory indene derivative designated chemically as (Z)-5-fluoro-2-methyl-1-[[ρ-(methylsulfinyl)phenyl]methylene]-1H-indene-3-acetic acid. It is not a salicylate, pyrazolone or propionic acid derivative. Its empirical formula is C20H17FO3S, with a molecular weight of 356.42. Sulindac, a yellow crystalline compound, is a weak organic acid practically insoluble in water below pH 4.5, but very soluble as the sodium salt or in buffers of pH 6 or higher.
Sulindac is available in 150 and 200 mg tablets for oral administration. Each tablet contains the following inactive ingredients: magnesium stearate, microcrystalline cellulose, plasdone and sodium starch glycolate.
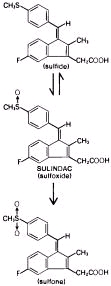
Following absorption, sulindac undergoes two major biotransformations - reversible reduction to the sulfide metabolite, and irreversible oxidation to the sulfone metabolite. Available evidence indicates that the biological activity resides with the sulfide metabolite.
The structural formulas of sulindac and its metabolites are:
CLINICAL PHARMACOLOGY
Pharmacodynamics
Sulindac is a non-steroidal anti-inflammatory drug (NSAID) that exhibits anti-inflammatory, analgesic and antipyretic activities in animal models. The mechanism of action, like that of other NSAIDs, is not completely understood but may be related to prostaglandin synthetase inhibition.
Absorption
The extent of sulindac absorption from sulindac tablets, USP is similar as compared to sulindac solution.
There is no information regarding food effect on sulindac absorption. Antacids containing magnesium hydroxide 200 mg and aluminum hydroxide 225 mg per 5 mL have been shown not to significantly decrease the extent of sulindac absorption.
| Pharmacokinetic Parameters for Normal and Healthy Patients | ||
| TABLE 1 | ||
| PHARMACOKINETIC PARAMETERS | NORMAL | ELDERLY |
| Tmax | Age 19-41 (n=24) (200 mg tablet) 3.38 ± 2.30 S 4.88 ± 2.57 SP 4.96 ± 2.36 SF (150 mg tablet) 3.90 ± 2.30 S 5.85 ± 4.49 SP 6.15 ± 3.07 SF | Age 65-87 (n=12) 400 mg qd 2.54 ± 1.52 S 5.75 ± 2.81 SF 6.83 ± 4.19 SP |
| Renal Clearance | (200 mg tablet) 68.12 ± 27.56 mL/min S 36.58 ± 12.61 mL/min SP (150 mg tablet) 74.39 ± 34.15 mL/min S 41.75 ± 13.72 mL/min SP | |
| Mean effective Half life (h) | 7.8 S 16.4 SF | |
| S = Sulindac SF = Sulindac Sulfide SP = Sulindac Sulfone | ||
Distribution
Sulindac, and its sulfone and sulfide metabolites, are 93.1, 95.4, and 97.9% bound to plasma proteins, predominantly to albumin. Plasma protein binding measured over a concentration range (0.5-2.0 µg/mL) was constant. Following an oral, radiolabeled dose of sulindac in rats, concentrations of radiolabel in red blood cells were about 10% of those in plasma. Sulindac penetrates the blood-brain and placental barriers. Concentrations in brain did not exceed 4% of those in plasma. Plasma concentrations in the placenta and in the fetus were less than 25% and 5% respectively, of systemic plasma concentrations. Sulindac is excreted in rat milk; concentrations in milk were 10 to 20% of those levels in plasma. It is not known if sulindac is excreted in human milk.
Metabolism
Sulindac undergoes two major biotransformations of its sulfoxide moiety: oxidation to the inactive sulfone and reduction to the pharmacologically active sulfide. The latter is readily reversible in animals and in man. These metabolites are present as unchanged compounds in plasma and principally as glucuronide conjugates in human urine and bile. A dihydroxydihydro analog has also been identified as a minor metabolite in human urine.
With the twice-a-day dosage regimen, plasma concentrations of sulindac and its two metabolites accumulate: mean concentration over a dosage interval at steady state relative to the first dose averages 1.5 and 2.5 times higher, respectively, for sulindac and its active sulfide metabolite.
Sulindac and its sulfone metabolite undergo extensive enterohepatic circulation relative to the sulfide metabolite in animals. Studies in man have also demonstrated that recirculation of the parent drug sulindac and its sulfone metabolite is more extensive than that of the active sulfide metabolite. The active sulfide metabolite accounts for less than six percent of the total intestinal exposure to sulindac and its metabolites.
Biochemical as well as pharmacological evidence indicates that the activity of sulindac resides in its sulfide metabolite. An in-vitro assay for inhibition of cyclooxygenase activity exhibited an EC50 of 0.02µM for sulindac sulfide. In-vivo models of inflammation indicate that activity is more highly correlated with concentrations of the metabolite than with parent drug concentrations.
Elimination
Approximately 50% of the administered dose of sulindac is excreted in the urine with the conjugated sulfone metabolite accounting for the major portion. Less than 1% of the administered dose of sulindac appears in the urine as the sulfide metabolite. Approximately 25% is found in the feces, primarily as the sulfone and sulfide metabolites.
The mean effective half life (T1/2) is 7.8 and 16.4 hours, respectively, for sulindac and its active sulfide metabolite.
Because sulindac is excreted in the urine primarily as biologically inactive forms, it may possibly affect renal function to a lesser extent than other non-steroidal anti-inflammatory drugs; however, renal adverse experiences have been reported with sulindac (see ADVERSE REACTIONS).
In a study of patients with chronic glomerular disease treated with therapeutic doses of sulindac, no effect was demonstrated on renal blood flow, glomerular filtration rate, or urinary excretion of prostaglandin E2 and the primary metabolite of prostacyclin, 6-keto-PGF1α. However, in other studies in healthy volunteers and patients with liver disease, sulindac was found to blunt the renal responses to intravenous furosemide, i.e., the diuresis, natriuresis, increments in plasma renin activity and urinary excretion of prostaglandins. These observations may represent a differentiation of the effects of sulindac on renal functions based on differences in pathogenesis of the renal prostaglandin dependence associated with differing dose-response relationships of different NSAIDs to the various renal functions influenced by prostaglandins (see PRECAUTIONS).
In healthy men, the average fecal blood loss, measured over a two-week period during administration of 400 mg per day of sulindac, was similar to that for placebo, and was statistically significantly less than that resulting from 4800 mg per day of aspirin.
Hepatic Insufficiency
Patients with acute and chronic hepatic disease may require reduced doses of sulindac compared to patients with normal hepatic function since hepatic metabolism is an important elimination pathway.
Following a single dose, plasma concentrations of the active sulfide metabolite have been reported to be higher in patients with alcoholic liver disease compared to healthy normal subjects.
Renal Insufficiency
Sulindac pharmacokinetics have been investigated in patients with renal insufficiency. The disposition of sulindac was studied in end-stage renal disease patients requiring hemodialysis. Plasma concentrations of sulindac and its sulfone metabolite were comparable to those of normal healthy volunteers whereas concentrations of the active sulfide metabolite were significantly reduced. Plasma protein binding was reduced and the AUC of the unbound sulfide metabolite was about half that in healthy subjects.
Sulindac and its metabolites are not significantly removed from the blood in patients undergoing hemodialysis.
Since sulindac is eliminated primarily by the kidneys, patients with significantly impaired renal function should be closely monitored.
A lower daily dosage should be anticipated to avoid excessive drug accumulation.
In controlled clinical studies sulindac was evaluated in the following five conditions:
1. Osteoarthritis
In patients with osteoarthritis of the hip and knee, the anti-inflammatory and analgesic activity of sulindac was demonstrated by clinical measurements that included: assessments by both patient and investigator of overall response; decrease in disease activity as assessed by both patient and investigator; improvement in ARA Functional Class; relief of night pain; improvement in overall evaluation of pain, including pain on weight bearing and pain on active and passive motion; improvement in joint mobility, range of motion, and functional activities; decreased swelling and tenderness; and decreased duration of stiffness following prolonged inactivity.
In clinical studies in which dosages were adjusted according to patient needs, sulindac, 200 to 400 mg daily was shown to be comparable in effectiveness to aspirin 2400 to 4800 mg daily. Sulindac was generally well tolerated, and patients on it had a lower overall incidence of total adverse effects, of milder gastrointestinal reactions, and of tinnitus than did patients on aspirin. (See ADVERSE REACTIONS)
2. Rheumatoid Arthritis
In patients with rheumatoid arthritis, the anti-inflammatory and analgesic activity of sulindac was demonstrated by clinical measurements that included: assessments by both patient and investigator of overall response; decrease in disease activity as assessed by both patient and investigator; reduction in overall joint pain; reduction in duration and severity of morning stiffness; reduction in day and night pain; decrease in time required to walk 50 feet; decrease in general pain as measured on a visual analog scale; improvement in the Ritchie articular index; decrease in proximal interphalangeal joint size; improvement in ARA Functional Class; increase in grip strength; reduction in painful joint count and score; reduction in swollen joint count and score; and increased flexion and extension of the wrist.
In clinical studies in which dosages were adjusted according to patient needs, sulindac 300 to 400 mg daily was shown to be comparable in effectiveness to aspirin 3600 to 4800 mg daily. Sulindac was generally well tolerated, and patients on it had a lower overall incidence of total adverse effects, of milder gastrointestinal reactions, and of tinnitus than did patients on aspirin. (See ADVERSE REACTIONS)
In patients with rheumatoid arthritis, sulindac may be used in combination with gold salts at usual dosage levels. In clinical studies, sulindac added to the regimen of gold salts usually resulted in additional symptomatic relief but did not alter the course of the underlying disease.
3. Ankylosing Spondylitis
In patients with ankylosing spondylitis, the anti-inflammatory and analgesic activity of sulindac was demonstrated by clinical measurements that included: assessments by both patient and investigator of overall response; decrease in disease activity as assessed by both patient and investigator; improvement in ARA Functional Class; improvement in patient and investigator evaluation of spinal pain, tenderness and/or spasm; reduction in the duration of morning stiffness; increase in the time to onset of fatigue; relief of night pain; increase in chest expansion; and increase in spinal mobility evaluated by fingers-to-floor distance, occiput to wall distance, the Schober Test, and the Wright Modification of the Schober Test. In a clinical study in which dosages were adjusted according to patient need, sulindac 200 to 400 mg daily was as effective as indomethacin 75 to 150 mg daily. In a second study, sulindac 300 to 400 mg daily was comparable in effectiveness to phenylbutazone 400 to 600 mg daily. Sulindac was better tolerated than phenylbutazone. (See ADVERSE REACTIONS)
4. Acute Painful Shoulder (Acute subacromial bursitis/supraspinatus tendinitis
In patients with acute painful shoulder (acute subacromial bursitis/supraspinatus tendinitis), the anti-inflammatory and analgesic activity of sulindac was demonstrated by clinical measurements that included: assessments by both patient and investigator of overall response; relief of night pain, spontaneous pain, and pain on active motion; decrease in local tenderness; and improvement in range of motion measured by abduction, and internal and external rotation. In clinical studies in acute painful shoulder, sulindac 300 to 400 mg daily and oxyphenbutazone 400 to 600 mg daily were shown to be equally effective and well tolerated.
5. Acute gouty arthritis
In patients with acute gouty arthritis, the anti-inflammatory and analgesic activity of sulindac was demonstrated by clinical measurements that included: assessments by both the patient and investigator of overall response; relief of weight-bearing pain; relief of pain at rest and on active and passive motion; decrease in tenderness; reduction in warmth and swelling; increase in range of motion; and improvement in ability to function. In clinical studies, sulindac at 400 mg daily and phenylbutazone at 600 mg daily were shown to be equally effective. In these short-term studies in which reduction of dosage was permitted according to response, both drugs were equally well tolerated.
INDICATIONS AND USAGE
Carefully consider the potential benefits and risks of sulindac and other treatment options before deciding to use sulindac. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS).
Sulindac is indicated for acute or long-term use in the relief of signs and symptoms of the following:
1. Osteoarthritis
2. Rheumatoid arthritis**
3. Ankylosing spondylitis
4. Acute painful shoulder (Acute subacromial bursitis/supraspinatus tendinitis)
5. Acute gouty arthritis
** The safety and effectivness of sulindac tablets have not been established in rheumatoid arthritis patients who are designated in the American Rheumatism Association classification as Functional Class IV (incapacitated, largely or wholly bedridden, or confined to wheelchair, little or no self-care).
CONTRAINDICATIONS
Sulindac is contraindicated in patients with known hypersensitivity to sulindac or the excipients (see DESCRIPTION).
Sulindac should not be given to patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs. Severe, rarely fatal, anaphylactic/anaphylactoid reactions to NSAIDs have been reported in such patients (see WARNINGS - Anaphylactic/Anaphylactoid Reactions and PRECAUTIONS - Preexisting Asthma).
Sulindac is contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery (see WARNINGS).
WARNINGS
Cardiovascular Thrombotic Events
Clinical trials of several COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of serious cardiovascular (CV) thrombotic events, myocardial infarction, and stroke, which can be fatal. All NSAIDs, both COX-2 selective and nonselective, may have a similar risk. Patients with known CV disease or risk factors for CV disease may be at greater risk. To minimize the potential risk for an adverse CV event in patients treated with an NSAID, the lowest effective dose should be used for the shortest duration possible. Physicians and patients should remain alert for the development of such events, even in the absence of previous CV symptoms. Patients should be informed about the signs and/or symptoms of serious CV events and the steps to take if they occur.
There is no consistent evidence that concurrent use of aspirin mitigates the increased risk of serious CV thrombotic events associated with NSAID use. The concurrent use of aspirin and an NSAID does increase the risk of serious GI events (see GI WARNINGS).
Two large, controlled, clinical trials of a COX-2 selective NSAID for the treatment of pain in the first 10-14 days following CABG surgery found an increased incidence of myocardial infarction and stroke (see CONTRAINDICATIONS).
Hypertension
NSAIDs, including sulindac, can lead to onset of new hypertension or worsening of pre-existing hypertension, either of which may contribute to the increased incidence of CV events. Patients taking thiazides or loop diuretics may have impaired response to these therapies when taking NSAIDs. NSAIDs, including sulindac, should be used with caution in patients with hypertension. Blood pressure (BP) should be monitored closely during the initiation of NSAID treatment and throughout the course of therapy.
Congestive Heart Failure and Edema
Fluid retention and edema have been observed in some patients taking NSAIDs. Sulindac should be used with caution in patients with fluid retention or heart failure.
Gastrointestinal Effects - Risk of Ulceration, Bleeding, and Perforation
NSAIDs, including sulindac, can cause serious gastrointestinal (GI) adverse events including inflammation, bleeding, ulceration, and perforation of the stomach, small intestine, or large intestine, which can be fatal. These serious adverse events can occur at any time, with or without warning symptoms, in patients treated with NSAIDs. Only one in five patients, who develop a serious upper GI adverse event on NSAID therapy, is symptomatic. Upper GI ulcers, gross bleeding, or perforation caused by NSAIDs occur in approximately 1% of patients treated for 3-6 months, and in about 2-4% of patients treated for one year. These trends continue with longer duration of use, increasing the likelihood of developing a serious GI event at some time during the course of therapy. However, even short-term therapy is not without risk.
NSAIDs should be prescribed with extreme caution in those with prior history of ulcer disease or gastrointestinal bleeding. Patients with a prior history of peptic ulcer disease and/or gastrointestinal bleeding who use NSAIDs have a greater than 10-fold increased risk for developing a GI bleed compared to patients with neither of these risk factors. Other factors that increase the risk for GI bleeding in patients treated with NSAIDs include concomitant use of oral corticosteroids or anticoagulants, longer duration of NSAID therapy, smoking, use of alcohol, older age, and poor general health status. Most spontaneous reports of fatal GI events are in elderly or debilitated patients and therefore, special care should be taken in treating this population.
To minimize the potential risk for an adverse GI event in patients treated with an NSAID, the lowest effective dose should be used for the shortest possible duration. Patients and physicians should remain alert for signs and symptoms of GI ulceration and bleeding during NSAID therapy and promptly initiate additional evaluation and treatment if a serious GI adverse event is suspected. This should include discontinuation of the NSAID until a serious GI adverse event is ruled out. For high risk patients, alternate therapies that do not involve NSAIDs should be considered.
Hepatic Effects
In addition to hypersensitivity reactions involving the liver, in some patients the findings are consistent with those of cholestatic hepatitis (see WARNINGS, Hypersensitivity). As with other non-steroidal anti-inflammatory drugs, borderline elevations of one or more liver tests without any other signs and symptoms may occur in up to 15% of patients taking NSAIDs including sulindac. These laboratory abnormalities may progress, may remain essentially unchanged, or may be transient with continued therapy. The SGPT (ALT) test is probably the most sensitive indicator of liver dysfunction. Meaningful (3 times the upper limit of normal) elevations of SGPT or SGOT (AST) occurred in controlled clinical trials in less than 1% of patients. Notable elevations of ALT or AST (approximately three or more times the upper limit of normal) have been reported in approximately 1% of patients in clinical trials with NSAIDs. In addition, rare cases of severe hepatic reactions, including jaundice and fatal fulminant hepatitis, liver necrosis and hepatic failure, some of them with fatal outcomes have been reported.
A patient with symptoms and/or signs suggesting liver dysfunction, or in whom an abnormal liver test has occurred, should be evaluated for evidence of the development of a more severe hepatic reaction while on therapy with sulindac. Although such reactions as described above are rare, if abnormal liver tests persist or worsen, if clinical signs and symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g., eosinophilia, rash, etc.), sulindac should be discontinued.
In clinical trials with sulindac, the use of doses of 600 mg/day has been associated with an increased incidence of mild liver test abnormalities (see DOSAGE AND ADMINISTRATION for maximum dosage recommendation).
Renal Effects
Long-term administration of NSAIDs has resulted in renal papillary necrosis and other renal injury. Renal toxicity has also been seen in patients in whom renal prostaglandins have a compensatory role in the maintenance of renal perfusion. In these patients, administration of a non-steroidal anti-inflammatory drug may cause a dose-dependent reduction in prostaglandin formation and, secondarily, in renal blood flow, which may precipitate overt renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, heart failure, liver dysfunction, those taking diuretics and ACE inhibitors, patients who are volume-depleted, and the elderly. Discontinuation of NSAID therapy is usually followed by recovery to the pretreatment state.
Advanced Renal Disease
No information is available from controlled clinical studies regarding the use of sulindac in patients with advanced renal disease. Therefore, treatment with sulindac is not recommended in these patients with advanced renal disease. If sulindac therapy must be initiated, close monitoring of the patient’s renal function is advisable.
Anaphylactic/Anaphylactoid Reactions
As with other NSAIDs, anaphylactic/anaphylactoid reactions may occur in patients without known prior exposure to sulindac. Sulindac should not be given to patients with the aspirin triad. This symptom complex typically occurs in asthmatic patients who experience rhinitis with or without nasal polyps, or who exhibit severe, potentially fatal bronchospasm after taking aspirin or other NSAIDs (see CONTRAINDICATIONS and PRECAUTIONS - Preexisting Asthma). Emergency help should be sought in cases where an anaphylactic/anaphylactoid reaction occurs.
Skin Reactions
NSAIDs, including sulindac, can cause serious adverse events such as exfoliative dermatitis, Stevens-Johnson Syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. These serious events may occur without warning. Patients should be informed about the signs and symptoms of serious skin manifestations and use of the drug should be discontinued at the first appearance of skin rash or any other sign of hypersensitivity.
Hypersensitivity
Rarely, fever and other evidence of hypersensitivity (see ADVERSE REACTIONS) including abnormalities in one or more liver function tests and severe skin reactions have occurred during therapy with sulindac. Fatalities have occurred in these patients. Hepatitis, jaundice, or both, with or without fever, may occur usually within the first one to three months of therapy. Determinations of liver function should be considered whenever a patient on therapy with sulindac develops unexplained fever, rash or other dermatologic reactions or constitutional symptoms. If unexplained fever or other evidence of hypersensitivity occurs, therapy with sulindac should be discontinued. The elevated temperature and abnormalities in liver function caused by sulindac characteristically have reverted to normal after discontinuation of therapy. Administration of sulindac should not be reinstituted in such patients.
PRECAUTIONS
General
Sulindac cannot be expected to substitute for corticosteroids or to treat corticosteroid insufficiency. Abrupt discontinuation of corticosteroids may lead to disease exacerbation. Patients on prolonged corticosteroid therapy should have their therapy tapered slowly if a decision is made to discontinue corticosteroids.
The pharmacological activity of sulindac in reducing fever and inflammation may diminish the utility of these diagnostic signs in detecting complications of presumed noninfectious, painful conditions.
Hematological Effects
Anemia is sometimes seen in patients receiving NSAIDs, including sulindac. This may be due to fluid retention, occult or gross GI blood loss, or an incompletely described effect upon erythropoiesis. Patients on long-term treatment with NSAIDs, including sulindac, should have their hemoglobin or hematocrit checked if they exhibit any signs or symptoms of anemia.
NSAIDs inhibit platelet aggregation and have been shown to prolong bleeding time in some patients. Unlike aspirin, their effect on platelet function is quantitatively less, of shorter duration, and reversible. Patients receiving sulindac who may be adversely affected by alterations in platelet function, such as those with coagulation disorders or patients receiving anticoagulants, should be carefully monitored.
Preexisting Asthma
Patients with asthma may have aspirin-sensitive asthma. The use of aspirin in patients with aspirin-sensitive asthma has been associated with severe bronchospasm which can be fatal. Since cross reactivity, including bronchospasm, between aspirin and other non-steroidal anti-inflammatory drugs has been reported in such aspirin-sensitive patients, sulindac should not be administered to patients with this form of aspirin sensitivity and should be used with caution in patients with preexisting asthma.
Renal Calculi
Sulindac metabolites have been reported rarely as the major or a minor component in renal stones in association with other calculus components. Sulindac should be used with caution in patients with a history of renal lithiasis, and they should be kept well hydrated while receiving sulindac.
Pancreatitis
Pancreatitis has been reported in patients receiving sulindac (see ADVERSE REACTIONS). Should pancreatitis be suspected, the drug should be discontinued and not restarted, supportive medical therapy instituted, and the patient monitored closely with appropriate laboratory studies (e.g., serum and urine amylase, amylase/creatinine clearance ratio, electrolytes, serum calcium, glucose, lipase, etc.). A search for other causes of pancreatitis as well as those conditions which mimic pancreatitis should be conducted.
Ocular Effects
Because of reports of adverse eye findings with non-steroidal anti-inflammatory agents, it is recommended that patients who develop eye complaints during treatment with sulindac have ophthalmologic studies.
Hepatic Insufficiency
In patients with poor liver function, delayed, elevated and prolonged circulating levels of the sulfide and sulfone metabolites may occur. Such patients should be monitored closely; a reduction of daily dosage may be required.
SLE and Mixed Connective Tissue Disease
In patients with systemic lupus erythematosus (SLE) and mixed connective tissue disease, there may be an increased risk of aseptic meningitis (see ADVERSE REACTIONS).
Information for Patients
Patients should be informed of the following information before initiating therapy with an NSAID and periodically during the course of ongoing therapy. Patients should also be encouraged to read the NSAID Medication Guide that accompanies each prescription dispensed.
- Sulindac, like other NSAIDs, may cause serious CV side effects, such as MI or stroke, which may result in hospitalization and even death. Although serious CV events can occur without warning symptoms, patients should be alert for the signs and symptoms of chest pain, shortness of breath, weakness, slurring of speech, and should ask for medical advice when observing any indicative sign or symptoms. Patients should be apprised of the importance of this follow-up (see WARNINGS, CARDIOVASCULAR EFFECTS).
- Sulindac, like other NSAIDs, can cause GI discomfort and, rarely, serious GI side effects, such as ulcers and bleeding, which may result in hospitalization and even death. Although serious GI tract ulcerations and bleeding can occur without warning symptoms, patients should be alert for the signs and symptoms of ulcerations and bleeding, and should ask for medical advice when observing any indicative sign or symptoms including epigastric pain, dyspepsia, melena, and hematemesis. Patients should be apprised of the importance of this follow-up (see WARNINGS, Gastrointestinal Effects - Risk of Ulceration, Bleeding, and Perforation).
- Sulindac, like other NSAIDs, can cause serious skin side effects such as exfoliative dermatitis, SJS, and TEN, which may result in hospitalizations and even death. Although serious skin reactions may occur without warning, patients should be alert for the signs and symptoms of skin rash and blisters, fever, or other signs of hypersensitivity such as itching, and should ask for medical advice when observing any indicative signs or symptoms. Patients should be advised to stop the drug immediately if they develop any type of rash and contact their physicians as soon as possible.
- Patients should promptly report signs or symptoms of unexplained weight gain or edema to their physicians.
- Patients should be informed of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, pruritus, jaundice, right upper quadrant tenderness, and “flu-like” symptoms). If these occur, patients should be instructed to stop therapy and seek immediate medical therapy.
- Patients should be informed of the signs of an anaphylactic/anaphylactoid reaction (e.g., difficulty breathing, swelling of the face or throat). If these occur, patients should be instructed to seek immediate emergency help (see WARNINGS).
- In late pregnancy, as with other NSAIDs, sulindac should be avoided because it may cause premature closure of the ductus arteriosus.
Laboratory Tests
Because serious GI tract ulcerations and bleeding can occur without warning symptoms, physicians should monitor for signs or symptoms of GI bleeding. Patients on long-term treatment with NSAIDs should have their CBC and a chemistry profile checked periodically. If clinical signs and symptoms consistent with liver or renal disease develop, systemic manifestations occur (e.g., eosinophilia, rash, etc.) or if abnormal liver tests persist or worsen, sulindac should be discontinued.
Ace Inhibitors and Angiotensin II Antagonists
Reports suggest that NSAIDs may diminish the antihypertensive effect of ACE-inhibitors and angiotensin II antagonists. These interactions should be given consideration in patients taking NSAIDs concomitantly with ACE-inhibitors or angiotensin II antagonists. In some patients with compromised renal function (e.g. elderly patients or patients who are volume-depleted, including those on diuretic therapy) who are being treated with non-steroidal anti-inflammatory drugs, the co-administration of an NSAID and an ACE-inhibitor or an angiotensin II antagonist may result in further deterioration of renal function, including possible acute renal failure, which is usually reversible. Therefore, monitor renal function periodically in patients receiving ACEIs or AIIAs and NSAIDs in combination therapy.
Acetaminophen
Acetaminophen had no effect on the plasma levels of sulindac or its sulfide metabolite.
Aspirin
The concomitant administration of aspirin with sulindac significantly depressed the plasma levels of the active sulfide metabolite. A double-blind study compared the safety and efficacy of sulindac 300 or 400 mg daily given alone or with aspirin 2.4 g/day for the treatment of osteoarthritis. The addition of aspirin did not alter the types of clinical or laboratory adverse experiences for sulindac; however, the combination showed an increase in the incidence of gastrointestinal adverse experiences. Since the addition of aspirin did not have a favorable effect on the therapeutic response to sulindac, the combination is not recommended.
Cyclosporine
Administration of non-steroidal anti-inflammatory drugs concomitantly with cyclosporine has been associated with an increase in cyclosporine-induced toxicity, possibly due to decreased synthesis of renal prostacyclin. NSAIDs should be used with caution in patients taking cyclosporine, and renal function should be carefully monitored.
Diflunisal
The concomitant administration of sulindac and diflunisal in normal volunteers resulted in lowering of the plasma levels of the active sulindac sulfide metabolite by approximately one-third.
Diuretics
Clinical studies, as well as post marketing observations, have shown that sulindac can reduce the natriuretic effect of furosemide and thiazides in some patients. This response has been attributed to inhibition of renal prostaglandin synthesis. During concomitant therapy with NSAIDs, the patient should be observed closely for signs of renal failure (see WARNINGS, Renal Effects), as well as to assure diuretic efficacy.
DMSO
DMSO should not be used with sulindac. Concomitant administration has been reported to reduce the plasma levels of the active sulfide metabolite and potentially reduce efficacy. In addition, this combination has been reported to cause peripheral neuropathy.
Lithium
NSAIDs have produced an elevation of plasma lithium levels and a reduction in renal lithium clearance. The mean minimum lithium concentration increased 15% and the renal clearance was decreased by approximately 20%. These effects have been attributed to inhibition of renal prostaglandin synthesis by the NSAID. Thus, when NSAIDs and lithium are administered concurrently, subjects should be observed carefully for signs of lithium toxicity.
Methotrexate
NSAIDs have been reported to competitively inhibit methotrexate accumulation in rabbit kidney slices. This may indicate that they could enhance the toxicity of methotrexate. Caution should be used when NSAIDs are administered concomitantly with methotrexate.
Oral anticoagulants
Although sulindac and its sulfide metabolite are highly bound to protein, studies in which sulindac was given at a dose of 400 mg daily have shown no clinically significant interaction with oral anticoagulants. However, patients should be monitored carefully until it is certain that no change in their anticoagulant dosage is required. Special attention should be paid to patients taking higher doses than those recommended and to patients with renal impairment or other metabolic defects that might increase sulindac blood levels. The effects of warfarin and NSAIDs on GI bleeding are synergistic, such that users of both drugs together have a risk of serious GI bleeding higher than users of either drug alone.
Oral hypoglycemic agents
Although sulindac and its sulfide metabolite are highly bound to protein, studies in which sulindac was given at a dose of 400 mg daily, have shown no clinically significant interaction with oral hypoglycemic agents. However, patients should be monitored carefully until it is certain that no change in their hypoglycemic dosage is required. Special attention should be paid to patients taking higher doses than those recommended and to patients with renal impairment or other metabolic defects that might increase sulindac blood levels.
Probenecid
Probenecid given concomitantly with sulindac had only a slight effect on plasma sulfide levels, while plasma levels of sulindac and sulfone were increased. Sulindac was shown to produce a modest reduction in the uricosuric action of probenecid, which probably is not significant under most circumstances.
Teratogenic Effects. Pregnancy Category C.
Reproductive studies conducted in rats and rabbits have not demonstrated evidence of developmental abnormalities. However, animal reproduction studies are not always predictive of human response. There are no adequate and well-controlled studies in pregnant women. Sulindac should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects
Because of the known effects of non-steroidal anti-inflammatory drugs on the fetal cardiovascular system (closure of ductus arteriosus), use during pregnancy (particularly late pregnancy) should be avoided.
The known effects of drugs of this class on the human fetus during the third trimester of pregnancy include: constriction of the ductus arteriosus prenatally, tricuspid incompetence, and pulmonary hypertension; non-closure of the ductus arteriosus postnatally which may be resistant to medical management; myocardial degenerative changes, platelet dysfunction with resultant bleeding, intracranial bleeding, renal dysfunction or failure, renal injury/dysgenesis which may result in prolonged or permanent renal failure, oligohydramnios, gastrointestinal bleeding or perforation, and increased risk of necrotizing enterocolitis.
In reproduction studies in the rat, a decrease in average fetal weight and an increase in numbers of dead pups were observed on the first day of the postpartum period at dosage levels of 20 and 40 mg/kg/day (2½ and 5 times the usual maximum daily dose in humans), although there was no adverse effect on the survival and growth during the remainder of the postpartum period. Sulindac prolongs the duration of gestation in rats, as do other compounds of this class. Visceral and skeletal malformations observed in low incidence among rabbits in some teratology studies did not occur at the same dosage levels in repeat studies, nor at a higher dosage level in the same species.
Labor and Delivery
In rat studies with NSAIDs, as with other drugs known to inhibit prostaglandin synthesis, an increased incidence of dystocia, delayed parturition, and decreased pup survival occurred. The effects of sulindac on labor and delivery in pregnant women are unknown.
Nursing Mothers
It is not known whether this drug is excreted in human milk; however, it is secreted in the milk of lactating rats. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from sulindac, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Geriatric Use
As with any NSAID, caution should be exercised in treating the elderly (65 years and older) since advancing age appears to increase the possibility of adverse reactions. Elderly patients seem to tolerate ulceration or bleeding less well than other individuals and many spontaneous reports of fatal GI events are in this population (see WARNINGS, Gastrointestinal Effects - Risk of Ulceration, Bleeding, and Perforation).
Sulindac is known to be substantially excreted by the kidney and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection and it may be useful to monitor renal function (See WARNINGS, Renal Effects).
ADVERSE REACTIONS
The following adverse reactions were reported in clinical trials or have been reported since the drug was marketed. The probability exists of a causal relationship between sulindac and these adverse reactions. The adverse reactions which have been observed in clinical trials encompass observations in 1,865 patients, including 232 observed for at least 48 weeks.
Incidence Greater Than 1%
Gastrointestinal
The most frequent types of adverse reactions occurring with sulindac are gastrointestinal; these include gastrointestinal pain (10%), dyspepsia***, nausea*** with or without vomiting, diarrhea***, constipation***, flatulence, anorexia and gastrointestinal cramps.
Dermatologic
Rash***, pruritus
Central Nervous System
Dizziness***, headache***, nervousness.
Special Senses
Tinnitus.
Miscellaneous
Edema (see WARNINGS).
Incidence Less Than 1 in 100
Gastrointestinal
Gastritis, gastroenteritis or colitis. Peptic ulcer and gastrointestinal bleeding have been reported. GI perforation and intestinal strictures (diaphragms) have been reported rarely.
Liver function abnormalities; jaundice, sometimes with fever; cholestasis; hepatitis; hepatic failure.
There have been rare reports of sulindac metabolites in common bile duct “sludge” and in biliary calculi in patients with symptoms of cholecystitis who underwent a cholecystectomy.
Pancreatitis (see PRECAUTIONS).
Ageusia; glossitis.
Dermatologic
Stomatitis, sore or dry mucous membranes, alopecia, photosensitivity.
Erythema multiforme, toxic epidermal necrolysis, Stevens-Johnson syndrome, and exfoliative dermatitis have been reported.
Cardiovascular
Congestive heart failure, especially in patients with marginal cardiac function; palpitation; hypertension.
Hematologic
Thrombocytopenia; ecchymosis, purpura, leukopenia, agranulocytosis, neutropenia, bone marrow depression, including aplastic anemia; hemolytic anemia, increased prothrombin time in patients on oral anticoagulants (see PRECAUTIONS).
Genitourinary
Urine discoloration; dysuria; vaginal bleeding, hematuria; proteinuria; crystalluria; renal impairment, including renal failure; interstitial nephritis; nephrotic syndrome.
Renal calculi containing sulindac metabolites have been observed rarely.
Metabolic
Hyperkalemia.
Musculoskeletal
Muscle weakness.
Psychiatric
Depression; psychic disturbances including acute psychosis.
Nervous System
Vertigo; insomnia; somnolence; paresthesia; convulsions; syncope; aseptic meningitis (especially in patients with systemic lupus erythematosus (SLE) and mixed connective tissue disease, see PRECAUTONS).
Special Senses
Blurred vision; visual disturbances; decreased hearing; metallic or bitter taste.
Respiratory
Epistaxis.
Hypersensitivity Reactions
Anaphylaxis; angioneurotic edema; urticaria; bronchial spasm; dyspnea.
Hypersensitivity vasculitis.
A potentially fatal apparent hypersensitivity syndrome has been reported. This syndrome may include constitutional symptoms (fever, chills, diaphoresis, flushing), cutaneous findings (rash or other dermatologic reactions – see above), conjunctivitis, involvement of major organs (changes in liver function including hepatic failure, jaundice, pancreatitis, pneumonitis with or without pleural effusion, leukopenia, leukocytosis, eosinophilia, disseminated intravascular coagulation, anemia, renal impairment, including renal failure), and other less specific findings (adenitis, arthralgia, arthritis, myalgia, fatigue, malaise, hypotension, chest pain, tachycardia).
Causal Relationship Unknown
A rare occurrence of fulminant necrotizing fasciitis, particularly in association with Group A β-hemolytic streptococcus, has been described in persons treated with non-steroidal anti-inflammatory agents, sometimes with fatal outcome (see also PRECAUTIONS, General).
Other reactions have been reported in clinical trials or since the drug was marketed, but occurred under circumstances where a causal relationship could not be established. However, in these rarely reported events, that possibility cannot be excluded. Therefore, these observations are listed to serve as alerting information to physicians.
Cardiovascular
Arrhythmia.
Metabolic
Hyperglycemia.
Nervous System
Neuritis.
Special Senses
Disturbances of the retina and its vasculature.
Miscellaneous
Gynecomastia.
___________________
***Incidence between 3% and 9%. Those reactions occurring in 1% to 3% of patients are not marked with an asterisk.
MANAGEMENT OF OVERDOSAGE
Cases of overdosage have been reported and rarely, deaths have occurred. The following signs and symptoms may be observed following overdosage; stupor, coma, diminished urine output and hypotension.
In the event of overdosage, the stomach should be emptied by inducing vomiting or by gastric lavage, and the patient carefully observed and given symptomatic and supportive treatment.
Animal studies show that absorption is decreased by the prompt administration of activated charcoal and excretion is enhanced by alkalinization of the urine.
DOSAGE AND ADMINISTRATION
Carefully consider the potential benefits and risks of sulindac and other treatment options before deciding to use sulindac. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS).
After observing the response to initial therapy with Sulindac Tablets USP, the dose and frequency should be adjusted to suit an individual patient’s needs.
Sulindac Tablets should be administered orally twice a day with food. The maximum dosage is 400 mg per day. Dosages above 400 mg per day are not recommended.
In osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis, the recommended starting dosage is 150 mg twice a day. The dosage may be lowered or raised depending on the response.
A prompt response (within one week) can be expected in about one-half of patients with osteoarthritis, ankylosing spondylitis, and rheumatoid arthritis. Others may require longer to respond.
In acute painful shoulder (acute subacromial bursitis/supraspinatus tendinitis) and acute gouty arthritis, the recommended dosage is 200 mg twice a day. After a satisfactory response has been achieved, the dosage may be reduced according to the response. In acute painful shoulder, therapy for 7-14 days is usually adequate. In acute gouty arthritis, therapy for 7 days is usually adequate.
HOW SUPPLIED
Sulindac Tablets USP, 200 mg are yellow, oval-shaped tablets, bisected and debossed with “Є” to the left of bisect and “11” to the right of bisect on one side, and plain on the other side, available in bottles of 60.
Storage
Store in a well-closed container at 20º to 25ºC (68 to 77ºF) [see USP Controlled Room Temperature].
Medication Guide
for
Non-Steroidal Antiinflammatory Drugs Drugs (NSAIDs)
(See the end of this Medication Guide for a list of prescription NSAID medicines)
What is the most important information I should know about medicines called Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)?
NSAID medicines may increase the chance of a heart attack or stroke that can lead to death.
This chance increases:
- with longer use of NSAID medicines
- in people who have heart disease
NSAID medicines should never be used right before or after heart surgery called a “coronary artery bypass graft (CABG).”
NSAID medicines can cause ulcers and bleeding in the stomach and intestines at any time during treatment.
Ulcers and bleeding:
- can happen without warning symptoms
- may cause death
The chance of a person getting an ulcer or bleeding increases with:
- taking medicines called “corticosteroids” and “anticoagulants”
- longer use
- smoking
- drinking alcohol
- older age
- having poor health
NSAID medicines should only be used:
- exactly as prescribed
- at the lowest dose possible for your treatment
- for the shortest time needed
___________________________________________________________________________
What are Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)?
NSAID medicines are used to treat pain and redness, swelling, and heat (inflammation) from medical conditions such as:
- different types of arthritis
- menstrual cramps and other types of short-term pain
Who should not take a Non-Steroidal Anti-Inflammatory Drug (NSAID)?
Do not take an NSAID medicine:
- if you had an asthma attack, hives, or other allergic reaction with aspirin or any other NSAID medicine
- for pain right before or after heart bypass surgery
Tell your healthcare provider:
- about all of your medical conditions.
- about all of the medicines you take. NSAIDs and some other medicines can interact with each other and cause serious side effects. Keep a list of your medicines to show to your
healthcare provider and pharmacist.
- if you are pregnant. NSAID medicines should not be used by pregnant women late in their pregnancy.
- if you are breastfeeding. Talk to your doctor.
What are the possible side effects of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)?
Serious side effects include:
| Other side effects include:
|
Get emergency help right away if you have any of the following symptoms:
|
|
Stop your NSAID medicine and call your healthcare provider right away if you have any of the following symptoms:
|
|
-
These are not all the side effects with NSAID medicines. Talk to your healthcare provider or pharmacist for more information about NSAID medicines.
-
Other information about Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
- Aspirin is an NSAID medicine but it does not increase the chance of a heart attack. Aspirin can cause bleeding in the brain, stomach, and intestines. Aspirin can also cause ulcers in the stomach and intestines.
- Some of these NSAID medicines are sold in lower doses without a prescription (over-the-
counter). Talk to your healthcare provider before using over-the-counter NSAIDs for more
than 10 days.
NSAID medicines that need a prescription
| Generic Name | Tradename |
| Celecoxib | Celebrex |
| Diclofenac | Cataflam, Voltaren, Arthrotec (combined with misoprostol) |
| Diflunisal | Dolobid |
| Etodolac | Lodine, Lodine XL |
| Fenoprofen | Nalfon, Nalfon 200 |
| Flurbiprofen | Ansaid |
| Ibuprofen | Motrin, Tab-Profen, Vicoprofen* (combined with hydrocodone), Combunox (combined with oxycodone) |
| Indomethacin | Indocin, Indocin SR, Indo-Lemmon, Indomethegan |
| Ketoprofen | Oruvail |
| Ketorolac | Toradol |
| Mefenamic Acid | Ponstel |
| Meloxicam | Mobic |
| Nabumetone | Relafen |
| Naproxen | Naprosyn, Anaprox, Anaprox DS, EC-Naprosyn, Naprelan, Naprapac (copackaged with lansoprazole) |
| Oxaprozin | Daypro |
| Piroxicam | Feldene |
| Sulindac | Clinoril |
| Tolmetin | Tolectin, Tolectin DS, Tolectin 600 |
| *Vicoprofen contains the same dose of ibuprofen as over-the-counter (OTC) NSAIDs, and is usually used for less than 10 days to treat pain. The OTC NSAID label warns that long term continuous use may increase the risk of heart attack or stroke. | |
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
- Manufactured By:
- Epic Pharma, LLC
- Laurelton, NY 11413
- Repackaged By:
- Aidarex Pharmaceuticals, LLC.
- Corona, CA 92880
- Revised November 2012
- MF018REV11/12
- OE1000