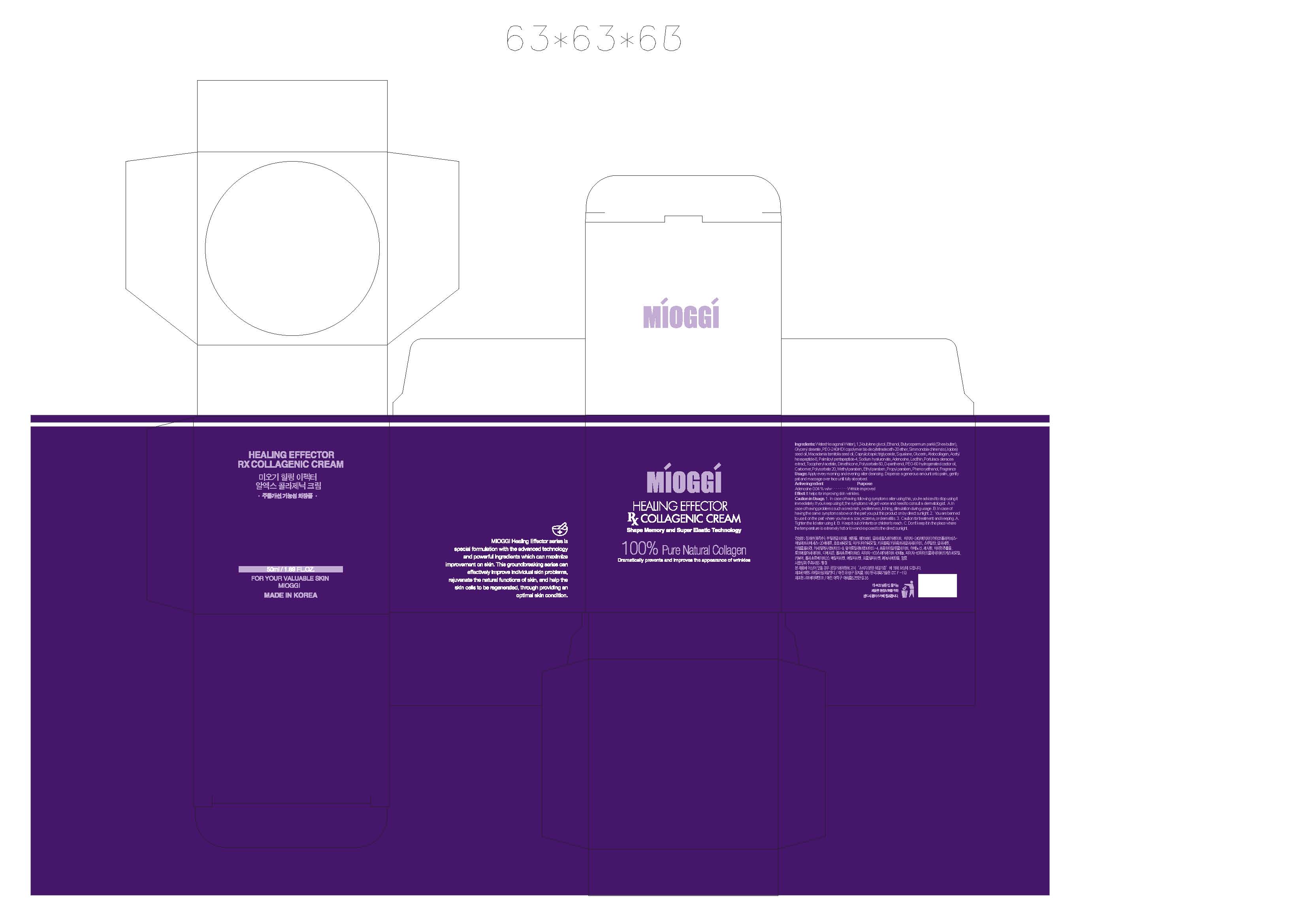
Water, Butylene glycol, Ethanol, Butyrospermum parkii (Shea Butter), Glyceryl stearate, PEG-240/HDI copolymer bis-decyltetradeceth-20 ether, Simmondsia chinensis (Jojoba) seed oil, Macadamia ternifolia seed oil, Caprylic/capric triglyceride, Squalane, Glycerin, Atelocollagen, Acetyl hexapeptide-8, Palmitoyl pentapeptide-4, Sodium hyaluronate, Adenosine, Lecithin, Portulaca oleracea extract, Tocopheryl acetate, Dimethicone, Polysorbate 60, D-panthenol, PEG-60 hydrogenated castor oil, Carbomer, Polysorbate 20, Methylparaben, Ethylparaben, Proplyparaben, Phenoxyethanol, Fragrance
apply every morning and evening at the end of basic care
gently spread appropriate amount over face using an outer circular motion until fully absorbed
gently spread appropriate amount over face using an outer circular motion until fully absorbed
1. In case of having following symptoms after using this, you're advised to stop using it immediately. If you keep using it, the symptoms will get worse and need to consult a dermatologist.
A. In case of having problems such as red rash, swollenness, itching, stimulation during usage.
B. In case of having the same symptoms above on the part you put this product on by direct sunlight.
2. You are banned to use it on the part where you have a scar, eczema, or dermatitis.
3. Caution for treatment and keeping.
A. Tighten the lid after using it.
B. Keep it out of infants or children's reach.
C. Don't keep it in the place where the temperature is extremely hot or low and exposed to the direct sunlight.
A. In case of having problems such as red rash, swollenness, itching, stimulation during usage.
B. In case of having the same symptoms above on the part you put this product on by direct sunlight.
2. You are banned to use it on the part where you have a scar, eczema, or dermatitis.
3. Caution for treatment and keeping.
A. Tighten the lid after using it.
B. Keep it out of infants or children's reach.
C. Don't keep it in the place where the temperature is extremely hot or low and exposed to the direct sunlight.