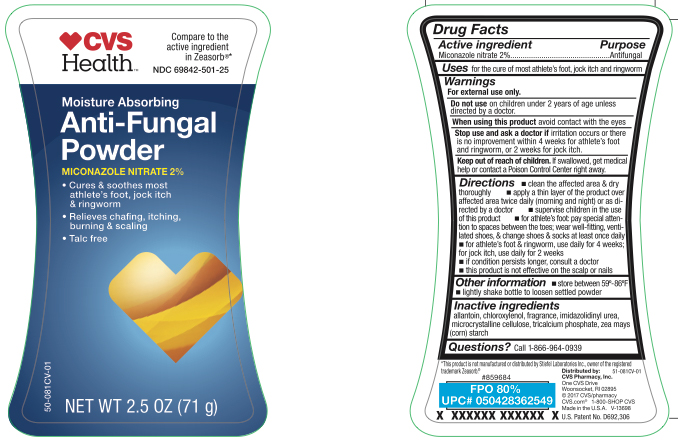
Active ingredient
Miconazole nitrate 2%
Uses
for the cure of most athlete's foot, jock itch and ringworm
Warnings
For external use only.
Do not use
on children 2 years of age unless directed by a doctor.
When using this product
avoid contact with the eyes
Stop and ask a doctor if
irritation occurs or there is no improvement within 4 weeks for athlete's foot and ringworm, or 2 weeks for jock itch.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away. Do not use on children under 2 years of age unless directed by a doctor.
Directions
- clean the affected area and dry thoroughly
- apply a thin layer of product over affected area twice daily (morning and night) or as directed by a doctor
- supervise children in the use of this product
- for athlete's foot: pay special attention to spaces between the toes; wear well-fitting, ventilated shoes, and change shoes and socks at least once daily
- for athlete's foot and ringworm, use daily for 4 weeks; for jock itch, use daily for 2 weeks
- if conditions persist longer, consult a doctor
- this product is not effective on the scalp or nails
Other information
- store between 59º - 86ºF
- lightly shake bottle to loosen settled powder
Inactive ingredients
allantoin, chloroxylenol, fragrance, imidazolidinyl urea, microcrystalline cellulose, tricalicum phosphate, zea mays (corn) starch
Questions?
call 1-866-964-0939
Principal Display Panel
CVS Health
Moisture Absorbing
Anti-Fungal Powder
MICONAZOLE NITRATE 2%
- Cures & soothes most athlete's foot, jock itch & ringworm
- Relieves chafing, itching, burning & scaling
- Talc free
NET WT 2.5 OZ (71 g)