ABUSE MISUSE AND ADDICTION
Amphetamine sulfate tablets has a high potential for abuse and misuse, which can lead to the development of a substance use disorder, including addiction. Misuse and abuse ofCNS stimulants, including amphetamine sulfate, can result in overdose and death (see OVERDOSAGE), and this risk is increased with higher doses orunapproved methods of administration. such as snorting or injection.
Before prescribing amphetamine sulfate tablets, assess each patient's risk for abuse, misuse, and addiction. Educate patients and their families about these risks, properstorage of the drug, and proper disposal of any unused drug. Throughoutamphetamine sulfate tablets treatment. reassess each patient's risk of abuse, misuse, andaddiction and frequently monitor for signs and symptoms of abuse, misuse, andaddiction (seeWARNINGSandDRUG ABUSE AND DEPENDENCE).
DESCRIPTION
Amphetamine Sulfate is a sympathomimetic amino of the amphetamine group. It is a white, odorless crystalline powder. It has a slightly bitter taste. Its solutions are acid to litmus, having a pH of 5.0 to 6.0. It is freely soluble in water and slightly soluble in alcohol.
Each tablet, for oral administration contains 5 mg or 10 mg of amphetamine sulfate. Each tablet also contains the following inactive ingredients: colloidal silicon dioxide, crospovidone, silicified microcrystalline cellulose and stearic acid. The 10 mg tablet also contains FD&C Blue #1 Aluminum Lake.
Structural Formula:
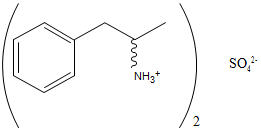
C18H28N2SO4 MW 368.49
CLINICAL PHARMACOLOGY
Amphetamines are non-catecholamine, sympathomimetic amines with CNS stimulant activity. Peripheral actions include elevations of systolic and diastolic blood pressures, and weak bronchodilator, and respiratory stimulant action.
Amphetamine, as the racemic form, differs from dextroamphetamine in a number of ways. The l-isomer is more potent than the d-isomer in cardiovascular activity, but much less potent in causing CNS excitatory effects. The racemic mixture also is less effective as an appetite suppressant when compared to dextroamphetamine. There is neither specific evidence which clearly establishes the mechanism whereby amphetamines produce mental and behavioral effects in children, nor conclusive evidence regarding how those effects relate to the condition of the central nervous system.
Drugs in this class used in obesity are commonly known as "anorectics" or "anorexigenics." It has not been established, however, that the action of such drugs in treating obesity is primarily one of appetite suppression. Other central nervous system actions or metabolic effects, may be involved, for example. Adult obese subjects instructed in dietary management and treated with "anorectic" drugs lose more weight on the average than these treated with placebo and diet, as determined in relatively short- term clinical trials.
The magnitude of increased weight loss of drug-treated patients over placebo-treated patients is only a fraction of a pound a week. The rate of weight loss is greatest in the first weeks of therapy for both drug and placebo subjects and tends to decrease in succeeding weeks. The origins of the increased weight loss due to the various possible drug effects are not established. The amount of weight loss associated with the use of an "anorectic" drug varies from trial to trial, and the increased weight loss appears to be related in part to variables other than the drug prescribed, such as the physician-investigator, the population treated, and the diet prescribed. Studies do not permit conclusions as to the relative importance of the drug and nondrug factors on weight loss.
The natural history of obesity is measured in years, whereas the studies cited are restricted to few weeks duration; thus, the total impact of drug-induced weight loss over that of diet alone must be considered clinically limited.
INDICATIONS AND USAGE
Amphetamine sulfate tablets are indicated for:
- Narcolepsy
- Attention Deficit Disorder with Hyperactivity as an integral part of a total treatment program which typically includes other remedial measures (psychological, educational, social) for a stabilizing effect in children with behavioral syndrome characterized by the following group of developmentally inappropriate symptoms: moderate to severe distractibility, short attention span, hyperactivity, emotional lability , and impulsivity. The diagnosis of the syndrome should not be made with finality when these symptoms are only of comparatively recent origin. Nonlocalizing (soft) neurological signs, learning disability, and abnormal EEG may or may not be present, and a diagnosis of central nervous system dysfunction may or not be warranted.
- Exogenous Obesity as a short term (a few weeks) adjunct in a regimen of weight reduction based on caloric restriction for patients refractory to alternative therapy, e.g., repeated diets, group programs, and other drugs. The limited usefulness of amphetamines (see CLINICAL PHARMACOLOGY) should be weighed against possible risks inherent in use of the drug, such as those described below.
CONTRAINDICATIONS
- Known hypersensitivity to amphetamine products
- During or within 14 days following the administration of monoamine oxidase inhibitors (hypertensive crises may result) (see WARNINGS).
WARNINGS
Abuse, Misuse, and Addiction
Amphetamine sulfate tablets has a high potential for abuse and misuse. The use of amphetamine sulfate tablets exposes individuals to the risks of abuse and misuse. which can lead to the development of a substance use disorder, including addiction. Amphetamine sulfate can be diverted for non-medical use into illicit channels or distribution (see DRUG ABUSE and DEPENDENCE). Misuse and abuse of CNS stimulants. including amphetamine sulfate tablets. can result in overdose and death (see OVER.DOSAGE). and this risk is increased with higher doses or unapproved methods of administration, such as snorting or injection.
Before prescribing amphetamine sulfate tablets, assess each patient’s risk for abuse, misuse, and addiction. Educate patients and their families about these risks and proper disposal of any unused drug. Advise patient to store amphetamine sulfate in a safe place. preferably locked, and instruct patients to not give amphetamine sulfate tablets to anyone else. Throughout amphetamine sulfate tablets treatment, reassess each patient’s risk of abuse, misuse. and addiction and frequently monitor for signs and symptoms of abuse, misuse. and addiction.
Risks to Patients with Serious Cardiac Disease
Sudden death has been reported in patients with structural cardiac abnormalities or other serious cardiac disease who are treated with CNS stimulants at the recommended ADHD dosages.
Avoid amphetamine sulfate tablets use in patients with known structural cardiac abnormalities,cardiomyopathy, serious cardiac arrhythmia, coronary artery disease, or other serious cardiac disease.
Increased Blood Pressure and Heart Rate
CNS stimulants cause an increase in blood pressure (mean increase about 2 to 4 mm Hg) and heart rare (mean increase about 3 to 6 bpm). Monitor all patients for potential tachycardia and hypertension.
Psychiatric Adverse Reactions
Exacerbation of Pre-Existing Psychosis
CNS stimulants may exacerbate symptoms of behavior disturbance and thought disorder in patients with a pre-existing psychotic disorder.
Induction of a Manic Episode in Patients with Bipolar Disorder
CNS stimulants may induce a manic or mixed episode in patients. Prior to initiating amphetamine sulfate tablets, screen patients for risk factors for developing a manic episode (e.g.,comorbid or history of depressive symptoms or a family history of suicide, bipolar disorder, or depression.
New Psychotic or Manic Symptoms
CNS stimulants, at recommended doses, may cause psychotic or manic symptom (e.g.,hallucinations, delusional thinking, or mania) in patients without a prior history of psychotic illness or mania). In a pooled analysis of multiple, short-term, placebo-controlled studies of CNS stimulants, psychotic or manic symptoms occurred in approximately 0.1% of CNS stimulant-treated patients, compared with 0% of placebo-treated patients. If such symptoms occur, consider discontinuing amphetamine sulfate tablets.
Long-Term Suppression of Growth in Pediatric Patients
CNS stimulants have been associated with weight loss and slowing of growth rate in Pediatric patients. Closely monitor growth (weight and height) in amphetamine sulfate tablets-treated pediatric patient treated with CNS stimulants.
Pediatric patients not growing or gaining height or weight as expected may need to have their treatment interrupted (see PRECAUTIONS , PEDIATRIC USE ).
Seizures
There is some clinical evidence that stimulants may lower the convulsive threshold in patients with prior history of seizures, in patients with prior EEG abnormalities in absence of seizures, and, very rarely, in patients without a history of seizures and no prior EEG evidence of seizures. In the presence of seizures, the drug should be discontinued.
Peripheral Vasculopathy, including Raynaud's phenomenon
Stimulants, including amphetamine sulfate tablets, used to treat ADHD are associated with peripheral vasculopathy, including Raynaud's phenomenon. Signs and symptoms are usually intermittent and mild; however, very rare sequelae include digital ulceration and/or soft tissue breakdown. Effects of peripheral vasculopathy, including Raynaud's phenomenon, were observed in post-marketing reports and at the therapeutic dosages of CNS stimulants in all age groups throughout the course of treatment. Signs and symptoms generally improved after dosage reduction or discontinuation of the CNS stimulant.
Careful observation for digital changes is necessary during amphetamine sulfate tablets treatment. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for patients who develop signs or symptoms of peripheral vasculopathy.
Serotonin Syndrome
Serotonin syndrome, a potentially life-threatening reaction, may occur when amphetamines are used in combination with other drugs that affect the serotonergic neurotransmitter systems such as monoamine oxidase inhibitors (MAOIs), selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, and St. John's Wort (see DRUGINTERACTIONS ). The co-administration of cytochrome P450 (CYP2D6) inhibitors may also increase the risk with increased exposure to amphetamine sulfate tablets. In these situations, consider an alternative non-serotonergic drug or an alternative drug that does not inhibit CYP2D6 (see DRUGINTERACTIONS).
Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures, and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea).
Concomitant use of amphetamine sulfate tablets with MAOI drugs is contraindicated (see CONTRAINDICATIONS ).
Discontinue treatment with amphetamine sulfate tablets and any concomitant serotonergic agents immediately if the above symptoms occur, and initiate supportive symptomatic treatment. If concomitant use of amphetamine sulfate tablets with other serotonergic drugs or CYP2D6 inhibitors is clinically warranted, initiate amphetamine sulfate tablets with lower doses, monitor patients for the emergence of serotonin syndrome during drug initiation or titration, and inform patients of the increased risk for serotonin syndrome.
Motor and Verbal Tics, and Worsening of Tourette's Syndrome
CNS stimulants, including amphetamine sulfate, have been associated with the onset orexacerbation of motor and verbal tics. Worsening of Tourette's syndrome has also beenreported. Assess the family history and clinically evaluate patients for tics or Tourette'ssyndrome before initiating amphetamine sulfate tablets. Regularly monitor patients for the emergence or worsening of tics or Tourette's syndrome with amphetamine sulfate tablets and discontinue treatment if clinically appropriate.
PRECAUTIONS
General
Caution is to be exercised in prescribing amphetamines for patients with even mild hypertension.
Information for Patients
Advise the patient to read the FDA-approved. patient labeling (Medication Guide).
Abuse, Misuse. and Addiction
Educate patients and their families about the risks of abuse, misuse, and addiction ofamphetamine sulfate tablets, which can lead to overdose and death, and proper disposal of any unused drug (see WARNINGS, DRUG ABUSE AND DEPENDENCE and OVERDOSAGE).
Advise patients to store amphetamine sulfate tablets in a safe place, preferably locked, and instruct patients to not give amphetamine sulfate tablets to anyone else.
Risks to Patients with Serious Cardiac Disease
Advise patients that there are potential risks to patients with serious cardiac disease, including sudden death. with amphetamine sulfate tablets use. Instruct patients to contact a healthcare provider immediately if they develop symptoms such as exertional chest pain, unexplained syncope, or other symptoms suggestive of cardiac disease (see WARNINGS).
Increased Blood Pressure and Heart Rate
Advise patients that amphetamine sulfate tablets can elevate blood pressure and heart rate (see WARNINGS)
Psychiatric Adverse Reactions
Advise patients that amphetamine sulfate tablets at recommended doses, can cause psychotic ormanic symptoms. even in patients without prior history of psychotic symptom or mania (see WARNINGS).
Long-Term Suppression of Growth in Pediatric Patients
Advise patients that amphetamine sulfate tablets, may cause slowing of growth including weight loss (see WARNINGS).
Circulation problems in fingers and toes [Peripheral vasculopathy, including Raynaud's phenomenon]
- Instruct patients beginning treatment with amphetamine sulfate tablets about the risk of peripheral vasculopathy, including Raynaud's Phenomenon, and associated signs and symptoms: fingers or toes may feel numb, cool, painful, and/or may change color from pale, to blue, to red.
- Instruct patients to report to their physician any new numbness, pain, skin color change, or sensitivity to temperature in fingers or toes.
- Instruct patients to call their physician immediately with any signs of unexplained wounds appearing on fingers or toes while taking amphetamine sulfate tablets.
- Further clinical evaluation (e.g., rheumatology referral) may be appropriate for certain patients.
Serotonin Syndrome
Caution patients about the risk of serotonin syndrome with concomitant use ofamphetamine sulfate tablets and other serotonergic drugs including SSRIs, SNRIs, triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan. buspirone. St. John's Wort. andwith drugs that impair metabolism of serotonin (in particular MAOIs, both those intendedto treat psychiatric disorders and also others such a linezolid (see CONTRAINDICATIONS, WARNINGS, and DRUG INTERACTIONS). Advise patients to contact their healthcare provider or report to the emergency room if they experience signs or symptoms of serotonin syndrome.
Motor and Verbal Tics, and Worsening of Tourette’s Syndrome
Advise patients that motor and verbal tics and worsening of Tourette's Syndrome mayoccur during treatment with amphetamine sulfate tablets. Instruct the patients to notify their healthcare provider if emergence or worsening of tics or Tourette 's syndrome occurs (see WARNINGS).
Amphetamines may impair the ability of the patient to engage in potentially hazardous activities such as operating machinery or vehicle: the patient should therefore be cautioned accordingly.
Drug Interactions
MAO inhibitors- MAOI antidepressants, as well as a metabolic of furazolidone, slowamphetamine metabolism. This slowing potentiates amphetamines. increasing their effecton the release of norepinephrine and other monoamines from adrenergic nerve endings:this can cause headaches and other signs of hypertensive crisis. A variety of neurologicaltoxic effect and malignant hyperpyrexia can occur, sometimes with fatal results.
Serotonergic Drugs - The concomitant use of amphetamine sulfate tablets and serotonergic drugsincreases the risk of serotonin syndrome. Initiate with lower doses and monitor patients forsigns and symptoms of serotonin syndrome, particularly during amphetamine sulfate tablets initiation or dosage increase. If serotonin syndrome occurs, discontinue amphetamine sulfate tablets and the concomitant serotonergic drug(s) (see WARNING and PRECAUTIONS).
CYP2D6 Inhibitors-The concomitant use of amphetamine sulfate tablets and CYP2D6 inhibitorsmay increase the exposure amphetamine sulfate tablets compared to the use of the drug alone andincrease the risk of serotonin syndrome. Initiate with lower doses and monitor patients forsigns and symptoms of serotonin syndrome particularly during amphetamine sulfate tablets initiation and after a dosage increase. If serotonin syndrome occurs. discontinue amphetamine sulfate tablets and the CYP2D6 inhibits) (see WARNING, OVERDOSAGE). Examples of CYP2D6 Inhibitor include paroxetine and fluoxetine (also serotonergic drugs), quinidine, ritonavir.
Acidifying agents - Gastrointestinal acidifying agents (guanethidine, reserpine, glutamic acid HCl, ascorbic acid, fruit juices, etc.) lower absorption of amphetamines. Urinary acidifying agents (ammonium chloride, sodium acid phosphate, etc.) increase concentration of the ionized species of the amphetamine molecule, thereby increasing urinary excretion. Both groups of agents lower blood levels and efficacy of amphetamines.
Adrenergic blockers - Adrenergic blockers are inhibited by amphetamines.
Alkalinizing agents - Gastrointestinal alkalinizing agents (sodium bicarbonate, etc.) increase absorption of amphetamines. Urinary alkalinizing agents (acetazolamide, some thiazides) increase the concentration of the nonionized species of the amphetamine molecule, thereby decreasing urinary excretion. Both groups of agents increase blood levels and therefore potentiate the action of amphetamines.
Antidepressants tricyclic - Amphetamines may enhance the activity of tricyclic or sympathomimetic agents; d-amphetamine with desipramine or protriptyline and possibly other tricyclics cause striking and sustained increases in the concentration of d- amphetamine in the brain; cardiovascular effects can be potentiated.
Antihistamines - Amphetamines may counteract the sedative effect of antihistamines.
Antihypertensives - Amphetamines may antagonize the hypotensive effects of antihypertensives.
Chlorpromazine - Chlorpromazine blocks dopamine and norepinephrine reuptake, thus inhibiting the central stimulant effects of amphetamine, and can be used to treat amphetamine poisoning.
Ethosuximide - Amphetamines may delay intestinal absorption of ethosuximide.
Haloperidol - Haloperidol blocks dopamine and norepinephrine reuptake, thus inhibiting the central stimulant affects of amphetamines.
Lithium carbonate - The antiobesity and stimulatory effects of amphetamines may be inhibited by lithium carbonate.
Meperidine - Amphetamines potentiate the analgesic effect of meperidine.
Methenamine therapy - Urinary excretion of amphetamines is increased, and efficacy is reduced by acidifying agents used in methenamine therapy.
Norepinephrine - Amphetamines enhance the adrenergic effect of norepinephrine.
Phenobarbital - Amphetamines may delay intestinal absorption of Phenobarbital. Co- administration of phenobarbital may produce a synergistic anticonvulsant action.
Phenytoin - Amphetamines may delay intestinal absorption of phenytoin; co- administration of phenytoin may produce a synergistic anticonvulsant action.
Propoxyphene - In cases of propoxyphene overdosage, amphetamine CNS stimulation is potentiated and fatal convulsions can occur.
Veratrum alkaloids - Amphetamines inhibit the hypotensive effect of veratrum alkaloids.
Drug/Laboratory Test interactions
Amphetamines can cause a significant elevation in plasma corticosteroid levels. This increase is greatest in the evening. Amphetamines may interfere with urinary steroid determinations.
Carcinogenesis/Mutagenesis
Mutagenicity studies and long term studies in animals to determine the carcinogenic potential of amphetamine sulfate have not been performed.
Pregnancy
Teratogenic Effects
Dextroamphetamine sulfate has been shown to have embryotoxic and teratogenic effects when administered to A/Jax mice and C57BL mice in doses approximately 41 times the maximum human dose. Embryotoxic effects were not seen in New Zealand white rabbits given the drug in doses 7 times the human dose nor in rats given 12.5 times the maximum human dose. There are no adequate and well-controlled studies in pregnant women. Amphetamine sulfate tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects
Infants born to mothers dependent on amphetamines have an increased risk of premature delivery and low birth weight. Also, these infants may experience symptoms of withdrawal as demonstrated by dysphoria, including agitation, and significant lassitude.
Nursing Mothers
Amphetamines are excreted in human milk. Mothers taking amphetamines should be advised to refrain from nursing.
Pediatric Use
Long-term effects of amphetamines in children have not been well established.
Amphetamines are not recommended for use as anorectic agents in children under 12 years of age, or in children under 3 years of age with Attention Deficit Disorder with Hyperactivity described under INDICATIONS AND USAGE.
Clinical experience suggests that in psychotic children, administration of amphetamines may exacerbate symptoms of behavior disturbance and thought disorder.
Data is inadequate to determine whether chronic administration of amphetamines may be associated with growth inhibition; therefore growth should be monitored during treatment. Drug Treatment is not indicated in all cases of Attention Deficit Disorder with Hyperactivity and should be considered only in light of the complete history and evaluation of the child. The decision to prescribe amphetamines should depend on the physician's assessment of the chronicity and severity of the child's symptoms and their appropriateness for his/her age. Prescription should not depend solely on the presence of one or more of the behavioral characteristics.
When these symptoms are associated with acute stress reactions, treatment with amphetamines is usually not indicated.
ADVERSE REACTIONS
Cardiovascular
Palpitations, tachycardia, elevation of blood pressure. There have been isolated reports of cardiomyopathy associated with chronic amphetamine use.
Central Nervous System
Psychotic episodes at recommended doses (rare), overstimulation, restlessness, dizziness, insomnia, euphoria, dyskinesia, dysphoria, tremor, headache, exacerbation of motor and verbal tics and Tourette's syndrome.
DRUG ABUSE AND DEPENDENCE
Controlled Substance
Amphetamine sulfate tablets contains amphetamine, a Schedule II controlled substance.
Abuse
Amphetamine sulfate tablets has a high potential for abuse and misuse which can lead to thedevelopment of a substance use disorder, including addiction (see WARNINGS).amphetamine sulfate tablets can be diverted for non-medical use into illicit channels or distribution.
Abuse is the intentional non-therapeutic use of a drug, even once, to achieve a desiredpsychological or physiological effect. Misuse is the intentional use. for therapeuticpurposes. of a drug by an individual in a way other than prescribed by a health careprovider or for whom it was not prescribed. Drug addiction is a cluster of behavioral,cognitive. and physiological phenomena that may include a strong desire to take the drug.difficulties in controlling drug use (e.g., continuing drug use despite harmfulconsequences. giving a higher priority to drug use than other activities and obligations).and possible tolerance or physical dependence.
Misuse and abuse of amphetamines may cause increased heart rate, respiratory rate, orblood pressure; sweating; dilated pupils; hyperactivity; restlessness; insomnia; decreasedappetite; loss of coordination; tremors: flushed skin; vomiting; and/or abdominal pain.Anxiety, psychosis, hostility, aggression, and suicidal or homicidal ideation have alsobeen observed with CNS stimulants abuse and/or misuse. Misuse and abuse of CNSstimulants, including amphetamine sulfate, can result in overdose and death (see OVERDOSAGE). and this risk is increased with higher doses or unapproved methods ofadministration. such as snorting or injection.
Dependence
Physical DependenceAmphetamine sulfate tablets may produce physical dependence. Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significantdose reduction of a drug.
Withdrawal signs and symptoms after abrupt discontinuation or dose reduction followingprolonged use of CNS stimulants including amphetamine sulfate tablets include dysphoric mood;depression; fatigue; vivid, unpleasant dreams; insomnia or hypersomnia; increasedappetite; and psychomotor retardation or agitation.
Tolerance
Amphetamine sulfate tablets may produce tolerance. Tolerance is a physiological state characterized by a reduced response to a drug after repeated administration (i.e., a higher dose of a drug is required to produce the same effect that was once obtained at a lower dose).
OVERDOSAGE
Clinical Effects of Overdose
Overdose of CNS stimulants is characterized by the following sympathomimetic effects:
- Cardiovascular effects including tachyarrhythmias, and hypertension or hypotension.Vasospasm, myocardial infarction, or aortic dissection may precipitate sudden cardiacdeath. Takotsubo cardiomyopathy may develop.
- CNS effects including psychomotor agitation, confusion, and hallucinations.Serotonin syndrome, seizures, cerebral vascular accidents, and coma may occur.
- Life-threatening hyperthermia (temperatures greater than 104°F) and rhabdomyolysismay develop.
Overdose Management
Consider the possibility of multiple drug ingestion. D-amphetamine is not dialyzable.Consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist foradditional overdose management recommendations
DOSAGE AND ADMINISTRATION
Regardless of indication, amphetamine should be administered at the lowest effective dosage and dosage should be individually adjusted. Late evening doses should be avoided because of resulting insomnia.
Narcolepsy
Usual dose is 5 to 60 milligrams per day in divided doses depending on the individual patient response.
Narcolepsy seldom occurs in children under 12 years of age; however, when it does, amphetamine sulfate tablets may be used. The suggested initial dose for patients aged 6 to 12 is 5 mg daily; daily dose may be raised in increments of 5 mg at weekly intervals until optimal response obtained. In patients 12 years of age and older, start with 10 mg daily; daily dosage may be raised in increments of 10 mg at weekly intervals until optimal response is obtained. If bothersome adverse reactions appear (e.g., insomnia or anorexia) dosage should be reduced. Give the first dose on awakening; additional doses (5 or 10 mg) at intervals of 4 to 6 hours.
Attention Deficit Disorder with Hyperactivity
Not recommended for children under 3 years of age.
In children from 3 to 5 years of age, start with 2.5 mg daily; daily dosage may be raised in increments of 2.5 mg at weekly intervals until optimal response is obtained.
In children 6 years of age or older, start with 5 mg once or twice daily; daily dosage may be raised in increments of 5 mg at weekly intervals until optimal response is obtained. Only in rare cases will it be necessary to exceed a total of 40 milligrams per day.
With tablets give first dose on awakening; additional doses (1 to 2) at intervals of 4 to 6 hours.
Where possible, drug administration should be interrupted occasionally to determine if there is a recurrence of behavioral symptoms sufficient to require continued therapy.
Prior to treating patients with Amphetamine Sulfate Tablets assess:
HOW SUPPLIED
Amphetamine sulfate tablets, USP is supplied as follows:
5 mg: White, round tablet, plain on one side and debossed "C/217" with a score on the other side in bottles of 100 tablets, NDC 43598-897-01.
10 mg: Blue, round tablet, plain on one side and double scores on the other side debossed with "C" in Quadrant 1, "2" in Quadrant 2, "1" in Quadrant 3 and "8" in Quadrant 4 in bottles of 100 tablets, NDC 43598-898-01.
Manufactured by:
Cerovene, Inc.
Valley Cottage, NY 10989
Distributed by:
Dr. Reddy's Laboratories Inc.
Princeton, NJ 08540, USA
Rev. 06/2023
| MEDICATION GUIDE
Amphetamine sulfate tablets, USP CII (am fet’ a meen sul’ fate) |
| What is the most important information I should know about amphetamine sulfate tablets?
Amphetamine sulfate tablet may cause serious side effects, including:
Call your healthcare provider or go to nearest hospital emergency room right away if you or your child have any signs of heart problems such as chest pain, shortness of breath, or fainting during treatment with amphetamine sulfate tablets.
Call your healthcare provider right away if you or your child have any new or worsening mental symptoms or problems during treatment with amphetamine sulfate tablets, especially hearing voices, seeing or believing things that are not real, or new manic symptoms. |
| What is amphetamine sulfate tablets?
Amphetamine sulfate tablets are a central nervous system (CNS) stimulant prescription medicine used for the treatment of:
It is not known Amphetamine sulfate tablets are safe and effective in children with exogenous obesity under 12 years of age.
|
Do not take amphetamine sulfate tablets if you or your child:
|
Before taking amphetamine sulfate tablets, tell your healthcare provider about all your or your child’s medical conditions, including if you or your child:
Amphetamine sulfate tablets and some medicines may interact with each other and cause serious side effects. Sometimes the doses of other medicines will need to be adjusted during treatment with amphetamine sulfate tablets. Your healthcare provider will decide if amphetamine sulfate tablets can be taken with other medicines. Especially tell your healthcare provider if you or your child takes:
Keep a list of your or your child’s medicines with you to show your healthcare provider and pharmacist when you or your child get a new medicine. Do not start any new medicine during treatment with amphetamine sulfate tablets without talking to your doctor first |
How should amphetamine sulfate tablets be taken?
|
| What should I avoid while taking amphetamine sulfate tablets? Do not drive, operate machinery, or do other dangerous activities until you know how amphetamine sulfate tablets affects you. |
| What are the possible side effects of amphetamine sulfate tablets?
Amphetamine sulfate tablets may cause serious side effects, including:
Call your healthcare provider right away if you or your child have any signs of unexplained wounds appearing on fingers or toe during treatment with amphetamine sulfate tablets
Call your doctor for medical advice about side effects. You may report side effects to Cerovene Inc. at 1-833-304-9569 or FDA at 1-800-FDA-1088. |
How should I store amphetamine sulfate tablets?
|
| General information about the safe and effective use of amphetamine sulfate tablets. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use amphetamine sulfate tablets for a condition for which it was not prescribed. Do not give amphetamine sulfate tablets to other people, even if they have the same symptoms that you are your child have.. It may harm them and it is against the law. You can ask your pharmacist or healthcare provider for information about amphetamine sulfate tablets that was written for healthcare professionals. |
| What are the ingredients in amphetamine sulfate tablets?
Active Ingredient: amphetamine sulfate Inactive Ingredients: colloidal silicon dioxide, crospovidone, silicified microcrystalline cellulose, and stearic acid. The 10 mg tablets also contain FD&C Blue #1 Aluminum Lake. Manufactured by: Cerovene Inc. Valley Cottage, NY 10989 Distributed by: Dr. Reddy's Laboratories Inc. Princeton, NJ 08540, USA For more information about amphetamine sulfate tablets, please contact Cerovene Inc. at 1-833-304-9569 |
|
|
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Rev. 06/2023

