ISOVUE 300
and 370 are NOT FOR INTRATHECAL USE.
See
Indications, and Dosage and Administration sections for further details
on proper use
DIAGNOSTIC
NONIONIC RADIOPAQUE CONTRAST MEDIA
For Angiography Throughout the Cardiovascular
System in Adults , Including Cerebral and Peripheral Arteriography,
Coronary Arteriography and Ventriculography, Selective Visceral Arteriography
and Aortography, Peripheral Venography (Phlebography), and in Pediatric
Patients for Angiocardiography;
or
For Intravenous
Adult and Pediatric
Computed Tomographic (CT) Imaging of
the Head and Body
DESCRIPTION
ISOVUE (Iopamidol Injection) is a stable,
aqueous, sterile, and nonpyrogenic solution for intravascular administration.
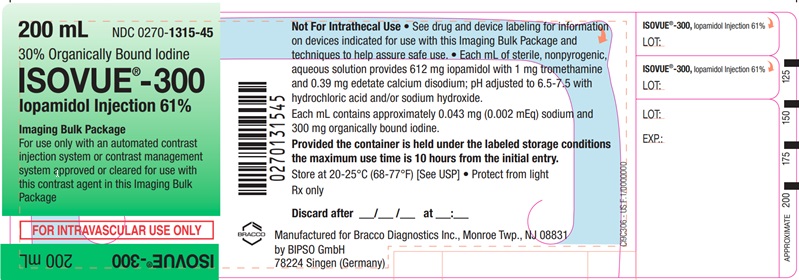
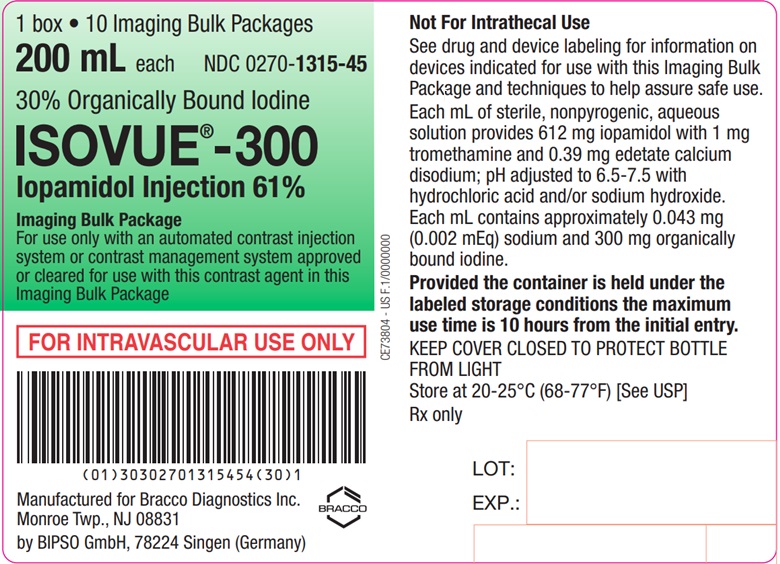
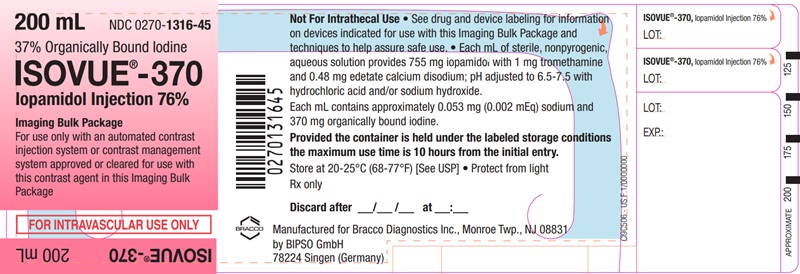
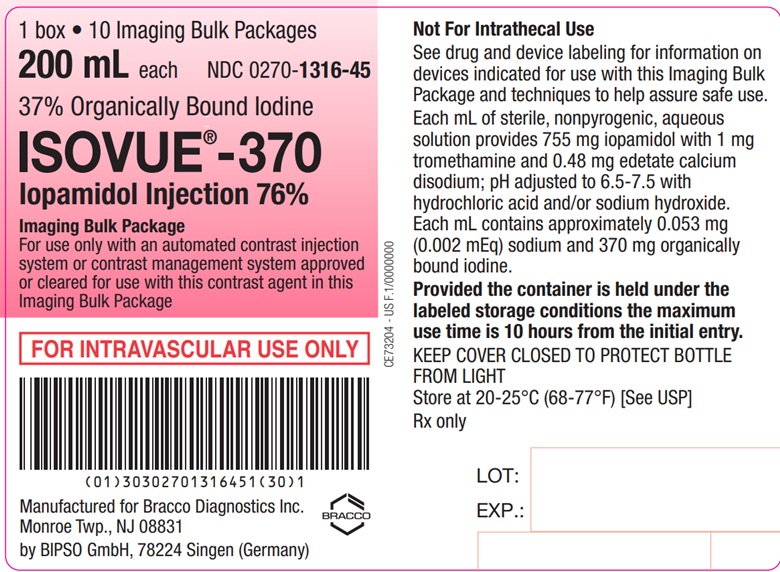
Each bottle is to be used as an Imaging Bulk Package for dispensing
multiple single doses of iopamidol injection for multiple patients,
using an automated contrast injection system, contrast management
system, or contrast media transfer set approved or cleared for use
with this contrast agent in this Imaging Bulk Package.
Each
mL of ISOVUE-300 (Iopamidol Injection 61%) provides 612 mg iopamidol
with 1 mg tromethamine and 0.39 mg edetate calcium disodium. The solution
contains approximately 0.043 mg (0.002 mEq) sodium and 300 mg organically
bound iodine per mL.
Each mL of ISOVUE-370 (Iopamidol
Injection 76%) provides 755 mg iopamidol with 1 mg tromethamine and
0.48 mg edetate calcium disodium. The solution contains approximately
0.053 mg (0.002 mEq) sodium and 370 mg organically bound iodine per
mL.
The pH of ISOVUE contrast media has been adjusted to 6.5-7.5 with hydrochloric acid and/or sodium hydroxide. Pertinent physicochemical data are noted below. ISOVUE (Iopamidol Injection) is hypertonic as compared to plasma and cerebrospinal fluid (approximately 285 and 301 mOsm/kg water, respectively).
| Iopamidol | ||
| Parameter | 61% | 76% |
| Concentration (mg iodine/mL) | 300 | 370 |
| Osmolality @ 37° C (mOsm/kg water) | 616 | 796 |
| Viscosity (cP) @ 37° C | 4.7 | 9.4 |
| @ 20° C | 8.8 | 20.9 |
| Specific Gravity @ 37° C | 1.339 | 1.405 |
Iopamidol is designated chemically as (S)-N,N’-bis[2-hydroxy-1-(hydroxymethyl)-ethyl]-2,4,6-triiodo-5-lactamidoisophthalamide. Structural formula:
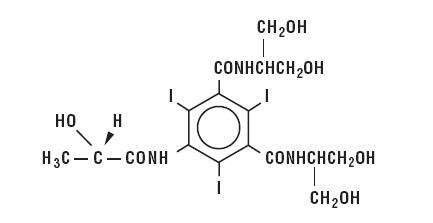 |
| MW 777.09 C17H22I3N3O8 CAS-60166-93-0 Organically Bound Iodine: 49% |
CLINICAL PHARMACOLOGY
Intravascular injection of a radiopaque diagnostic agent opacifies
those vessels in the path of flow of the contrast medium, permitting
radiographic visualization of the internal structures of the human
body until significant hemodilution occurs.
Following
intravascular injection, radiopaque diagnostic agents are immediately
diluted in the circulating plasma. Calculations of apparent volume
of distribution at steady-state indicate that iopamidol is distributed
between the circulating blood volume and other extracellular fluid;
there appears to be no significant deposition of iopamidol in tissues.
Uniform distribution of iopamidol in extracellular fluid is reflected
by its demonstrated utility in computed tomographic imaging of the
head and body following intravenous administration.
The
pharmacokinetics of intravenously administered iopamidol in normal
subjects conforms to an open two-compartment model with first order
elimination (a rapid alpha phase for drug distribution and a slow
beta phase for drug elimination). The elimination serum or plasma
half-life is approximately two hours; the half-life is not dose dependent.
No significant metabolism, deiodination, or biotransformation occurs.
Iopamidol is excreted mainly through the kidneys following intravascular
administration. In patients with impaired renal function, the elimination
half-life is prolonged dependent upon the degree of impairment. In
the absence of renal dysfunction, the cumulative urinary excretion
for iopamidol, expressed as a percentage of administered intravenous
dose, is approximately 35 to 40 percent at 60 minutes, 80 to 90 percent
at 8 hours, and 90 percent or more in the 72- to 96-hour period after
administration. In normal subjects, approximately one percent or less
of the administered dose appears in cumulative 72- to 96-hour fecal
specimens.
ISOVUE may be visualized in the renal parenchyma
within 30-60 seconds following rapid intravenous administration. Opacification
of the calyces and pelves in patients with normal renal function becomes
apparent within 1 to 3 minutes, with optimum contrast occurring between
5 and 15 minutes. In patients with renal impairment, contrast visualization
may be delayed.
Iopamidol displays little tendency to
bind to serum or plasma proteins.
No evidence of in vivo
complement activation has been found in normal subjects.
Animal studies indicate that iopamidol does not cross the blood-brain
barrier to any significant extent following intravascular administration.
ISOVUE (Iopamidol Injection) enhances computed tomographic brain
imaging through augmentation of radiographic efficiency. The degree
of enhancement of visualization of tissue density is directly related
to the iodine content in an administered dose; peak iodine blood levels
occur immediately following rapid injection of the dose. These levels
fall rapidly within five to ten minutes. This can be accounted for
by the dilution in the vascular and extracellular fluid compartments
which causes an initial sharp fall in plasma concentration. Equilibration
with the extracellular compartments is reached in about ten minutes,
thereafter, the fall becomes exponential. Maximum contrast enhancement
frequently occurs after peak blood iodine levels are reached. The
delay in maximum contrast enhancement can range from five to forty
minutes depending on the peak iodine levels achieved and the cell
type of the lesion. This lag suggests that radiographic contrast enhancement
is at least in part dependent on the accumulation of iodine within
the lesion and outside the blood pool, although the mechanism by which
this occurs is not clear. The radiographic enhancement of nontumoral
lesions, such as arteriovenous malformations and aneurysms, is probably
dependent on the iodine content of the circulating blood pool.
In CT head imaging, ISOVUE (Iopamidol Injection) does not accumulate
in normal brain tissue due to the presence of the “blood-brain” barrier.
The increase in x-ray absorption in normal brain is due to the presence
of contrast agent within the blood pool. A break in the blood-brain
barrier such as occurs in malignant tumors of the brain allows the
accumulation of the contrast medium within the interstitial tissue
of the tumor. Adjacent normal brain tissue does not contain the contrast
medium.
In nonneural tissues (during computed tomography
of the body), iopamidol diffuses rapidly from the vascular into the
extravascular space. Increase in x-ray absorption is related to blood
flow, concentration of the contrast medium, and extraction of the
contrast medium by interstitial tissue of tumors since no barrier
exists. Contrast enhancement is thus due to the relative differences
in extravascular diffusion between normal and abnormal tissue, quite
different from that in the brain.
The pharmacokinetics
of iopamidol in both normal and abnormal tissue have been shown to
be variable. Contrast enhancement appears to be greatest soon after
administration of the contrast medium, and following intraarterial
rather than intravenous administration. Thus, greatest enhancement
can be detected by a series of consecutive two- to three-second scans
performed just after injection (within 30 to 90 seconds), i.e., dynamic
computed tomographic imaging.
INDICATIONS AND USAGE
ISOVUE (Iopamidol Injection) is indicated for angiography throughout the cardiovascular system in adults, including cerebral and peripheral arteriography, coronary arteriography and ventriculography, selective visceral arteriography and aortography, peripheral venography (phlebography), and in pediatric patients for angiocardiography; or for intravenous use in adult and pediatric for computed tomographic (CT) imaging of the head and body (see below).
CT Head Imaging
ISOVUE
may be used to refine diagnostic precision in areas of the brain which
may not otherwise have been satisfactorily visualized.
Tumors
ISOVUE may
be useful to investigate the presence and extent of certain malignancies
such as: gliomas including malignant gliomas, glioblastomas, astrocytomas,
oligodendrogliomas and gangliomas, ependymomas, medulloblastomas,
meningiomas, neuromas, pinealomas, pituitary adenomas, craniopharyngiomas,
germinomas, and metastatic lesions. The usefulness of contrast enhancement
for the investigation of the retrobulbar space and in cases of low
grade or infiltrative glioma has not been demonstrated.
In calcified lesions, there is less likelihood of enhancement.
Following therapy, tumors may show decreased or no enhancement.
The opacification of the inferior vermis following contrast media
administration has resulted in false-positive diagnosis in a number
of otherwise normal studies.
Nonneoplastic Conditions
ISOVUE may be beneficial
in the image enhancement of nonneoplastic lesions. Cerebral infarctions
of recent onset may be better visualized with contrast enhancement,
while some infarctions are obscured if contrast media are used. The
use of iodinated contrast media results in contrast enhancement in
about 60 percent of cerebral infarctions studied from one to four
weeks from the onset of symptoms.
Sites of active infection
may also be enhanced following contrast media administration.
Arteriovenous malformations and aneurysms will show contrast enhancement.
For these vascular lesions, the enhancement is probably dependent
on the iodine content of the circulating blood pool.
Hematomas
and intraparenchymal bleeders seldom demonstrate any contrast enhancement.
However, in cases of intraparenchymal clot, for which there is no
obvious clinical explanation, contrast media administration may be
helpful in ruling out the possibility of associated arteriovenous
malformation.
CT Body Imaging
ISOVUE (Iopamidol Injection) may be used for enhancement
of computed tomographic images for detection and evaluation of lesions
in the liver, pancreas, kidneys, aorta, mediastinum, abdominal cavity,
pelvis and retroperitoneal space.
Enhancement of computed
tomography with ISOVUE may be of benefit in establishing diagnoses
of certain lesions in these sites with greater assurance than is possible
with CT alone, and in supplying additional features of the lesions
(e.g., hepatic abscess delineation prior to percutaneous drainage).
In other cases, the contrast agent may allow visualization of lesions
not seen with CT alone (e.g. tumor extension), or may help to define
suspicious lesions seen with unenhanced CT (e.g., pancreatic cyst).
Contrast enhancement appears to be greatest within 60 to 90 seconds
after bolus administration of contrast agent. Therefore, utilization
of a continuous scanning technique (“dynamic CT scanning”) may improve
enhancement and diagnostic assessment of tumor and other lesions such
as an abscess, occasionally revealing unsuspected or more extensive
disease. For example, a cyst may be distinguished from a vascularized
solid lesion when precontrast and enhanced scans are compared; the
nonperfused mass shows unchanged x-ray absorption (CT number). A vascularized
lesion is characterized by an increase in CT number in the few minutes
after a bolus of intravascular contrast agent; it may be malignant,
benign, or normal tissue, but would probably not be a cyst, hematoma,
or other nonvascular lesion.
Because unenhanced scanning
may provide adequate diagnostic information in the individual patient,
the decision to employ contrast enhancement, which may be associated
with risk and increased radiation exposure, should be based upon a
careful evaluation of clinical, other radiological, and unenhanced
CT findings.
WARNINGS
Severe Adverse Events-lnadvertent
Intrathecal Administration
Serious adverse
reactions have been reported due to the inadvertent intrathecal administration
of iodinated contrast media that are not indicated for intrathecal
use.
These serious adverse reactions include:
death, convulsions, cerebral hemorrhage, coma, paralysis, arachnoiditis,
acute renal failure, cardiac arrest, seizures, rhabdomyolysis, hyperthermia,
and brain edema. Special attention must be given to insure that this
drug product is not inadvertently administered intrathecally.
General
Nonionic iodinated contrast media inhibit blood coagulation, in vitro, less than ionic contrast media. Clotting has been reported when blood remains in contact with syringes containing nonionic contrast media.
Serious, rarely fatal, thromboembolic events causing myocardial infarction and stroke have been reported during angiographic procedures with both ionic and nonionic contrast media. Therefore, meticulous intravascular administration technique is necessary, particularly during angiographic procedures, to minimize thromboembolic events. Numerous factors, including length of procedure, catheter and syringe material, underlying disease state, and concomitant medications may contribute to the development of thromboembolic events. For these reasons, meticulous angiographic techniques are recommended including close attention to guidewire and catheter manipulation, use of manifold systems and/or three way stopcocks, frequent catheter flushing with heparinized saline solutions, and minimizing the length of the procedure. The use of plastic syringes in place of glass syringes has been reported to decrease but not eliminate the likelihood of in vitro clotting.
Caution must be exercised in patients with severely impaired renal function, those with combined renal and hepatic disease, or anuria, particularly when larger doses are administered.
Radiopaque diagnostic contrast agents are potentially hazardous in patients with multiple myeloma or other paraproteinemia, particularly in those with therapeutically resistant anuria. Myeloma occurs most commonly in persons over age 40. Although neither the contrast agent nor dehydration has been proved separately to be the cause of anuria in myelomatous patients, it has been speculated that the combination of both may be causative. The risk in myelomatous patients is not a contraindication; however, special precautions are required.
Contrast media may promote sickling in individuals who are homozygous for sickle cell disease when injected intravenously or intraarterially.
Administration of radiopaque materials to patients known or suspected of having pheochromocytoma should be performed with extreme caution. If, in the opinion of the physician, the possible benefits of such procedures outweigh the considered risks, the procedures may be performed; however, the amount of radiopaque medium injected should be kept to an absolute minimum. The blood pressure should be assessed throughout the procedure and measures for treatment of a hypertensive crisis should be available. These patients should be monitored very closely during contrast enhanced procedures.
Reports of thyroid storm following the use of iodinated radiopaque diagnostic agents in patients with hyperthyroidism or with an autonomously functioning thyroid nodule suggest that this additional risk be evaluated in such patients before use of any contrast medium.
Thyroid Dysfunction in Pediatric Patients 0 to 3 Years of Age: Thyroid dysfunction characterized by hypothyroidism or transient thyroid suppression has been reported after both single exposure and multiple exposures to iodinated contrast media in pediatric patients 0 to 3 years of age.
Younger age, very low birth weight, prematurity, underlying medical conditions affecting thyroid function, admission to neonatal or pediatric intensive care units, and congenital cardiac conditions are associated with an increased risk of hypothyroidism after ICM exposure. Pediatric patients with congenital cardiac conditions may be at greatest risk given that they often require high doses of contrast during invasive cardiac procedures.
An underactive thyroid during early life may be harmful for cognitive and neurological development and may require thyroid hormone replacement therapy. After exposure to ICM, individualize thyroid function monitoring based on underlying risk factors, especially in term and preterm neonates.
Severe Cutaneous Adverse Reactions: Severe cutaneous adverse reactions (SCAR) may develop from 1 hour to several weeks after intravascular contrast agent administration. These reactions include Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), acute generalized exanthematous pustulosis (AGEP) and drug reaction with eosinophilia and systemic symptoms (DRESS). Reaction severity may increase and time to onset may decrease with repeat administration of contrast agent; prophylactic medications may not prevent or mitigate severe cutaneous adverse reactions. Avoid administering ISOVUE to patients with a history of a severe cutaneous adverse reaction to ISOVUE.
PRECAUTIONS
General
Diagnostic procedures
which involve the use of any radiopaque agent should be carried out
under the direction of personnel with the prerequisite training and
with a thorough knowledge of the particular procedure to be performed.
Appropriate facilities should be available for coping with any complication
of the procedure, as well as for emergency treatment of severe reaction
to the contrast agent itself. After parenteral administration of a
radiopaque agent, competent personnel and emergency facilities should
be available for at least 30 to 60 minutes since severe delayed reactions
may occur. Caution should be exercised in hydrating patients with
underlying conditions that may be worsened by fluid overload, such
as congestive heart failure.
Preparatory dehydration is
dangerous and may contribute to acute renal failure in patients with
advanced vascular disease, diabetic patients, and in susceptible nondiabetic
patients (often elderly with preexisting renal disease). Patients
should be well hydrated prior to and following iopamidol administration.
The possibility of a reaction, including serious, life-threatening,
fatal, anaphylactoid or cardiovascular reactions, should always be
considered (see ADVERSE REACTIONS). Patients at increased risk include those with a history of a previous
reaction to a contrast medium, patients with a known sensitivity to
iodine per se, and patients with a known clinical hypersensitivity
(bronchial asthma, hay fever, and food allergies). The occurrence
of severe idiosyncratic reactions has prompted the use of several
pretesting methods. However, pretesting cannot be relied upon to predict
severe reactions and may itself be hazardous for the patient. It is
suggested that a thorough medical history with emphasis on allergy
and hypersensitivity, prior to the injection of any contrast medium,
may be more accurate than pretesting in predicting potential adverse
reactions. A positive history of allergies or hypersensitivity does
not arbitrarily contraindicate the use of a contrast agent where a
diagnostic procedure is thought essential, but caution should be exercised.
Premedication with antihistamines or corticosteroids to avoid or minimize
possible allergic reactions in such patients should be considered.
Recent reports indicate that such pretreatment does not prevent serious
life-threatening reactions but may reduce both their incidence and
severity.
Pre-existing conditions, such as pacemakers
or cardiac medications, specifically beta-blockers, may mask or alter
the signs or symptoms of an anaphylactoid reaction, as well as masking
or altering the response to particular medications used for treatment.
For example, beta-blockers inhibit a tachycardiac response, and can
lead to the incorrect diagnosis of a vasovagal rather than an anaphylactoid
reaction. Special attention to this possibility is particularly critical
in patients suffering from serious, life-threatening reactions.
General anesthesia may be indicated in the performance of some
procedures in selected patients; however, a higher incidence of adverse
reactions has been reported with radiopaque media in anesthetized
patients, which may be attributable to the inability of the patient
to identify untoward symptoms, or to the hypotensive effect of anesthesia
which can reduce cardiac output and increase the duration of exposure
to the contrast agent.
Even though the osmolality of iopamidol
is low compared to diatrizoate or iothalamate based ionic agents of
comparable iodine concentration, the potential transitory increase
in the circulatory osmotic load in patients with congestive heart
failure requires caution during injection. These patients should be
observed for several hours following the procedure to detect delayed
hemodynamic disturbances. Injection site pain and swelling may occur.
In the majority of cases it is due to extravasation of contrast medium.
Reactions are usually transient and recover without sequelae. However,
inflammation and even skin necrosis have been seen on very rare occasions.
In angiographic procedures, the possibility of dislodging plaques
or damaging or perforating the vessel wall, or inducing vasospasm,
and or subsequent ischemic events, should be borne in mind during
catheter manipulations and contrast medium injection. Test injections
to ensure proper catheter placement are suggested.
Selective coronary arteriography should be performed only
in selected patients and those in whom the expected benefits outweigh
the procedural risk. The inherent risks of angiocardiography in patients with chronic pulmonary emphysema must be weighed against
the necessity for performing this procedure. Angiography should be
avoided whenever possible in patients with homocystinuria, because
of the risk of inducing thrombosis and embolism. See also Pediatric Use.
In addition to
the general precautions previously described, special care is required
when venography is performed in patients with suspected thrombosis,
phlebitis, severe ischemic disease, local infection or a totally obstructed
venous system.
Extreme caution during injection of contrast
media is necessary to avoid extravasation and fluoroscopy is recommended.
This is especially important in patients with severe arterial or venous
disease.
Information for Patients
Patients receiving injectable radiopaque diagnostic agents should be instructed to:
- Inform your physician if you are pregnant.
- Inform your physician if you are diabetic or if you have multiple myeloma, pheochromocytoma, homozygous sickle cell disease, or known thyroid disorder (see WARNINGS).
- Inform your physician if you are allergic to any drugs, food, or if you had any reactions to previous injections of substances used for x-ray procedures (see PRECAUTIONS-General).
- Inform your physician about any other medications you are currently taking, including nonprescription drugs, before you have this procedure.
- Advise patients to inform their physician if they develop a rash after receiving Isovue.
- Advise parents/caregivers about the risk of developing thyroid dysfunction after ISOVUE administration. Advise parents/caregivers about when to seek medical care for their child to monitor for thyroid function (see WARNINGS).
Drug Interactions
Renal toxicity has been reported in a few
patients with liver dysfunction who were given oral cholecystographic
agents followed by intravascular contrast agents. Administration of
intravascular agents should therefore be postponed in any patient
with a known or suspected hepatic or biliary disorder who has recently
received a cholecystographic contrast agent.
Other drugs
should not be admixed with iopamidol.
Drug/Laboratory Test Interactions
The results of PBI and radioactive iodine uptake studies,
which depend on iodine estimations, will not accurately reflect thyroid
function for up to 16 days following administration of iodinated contrast
media. However, thyroid function tests not depending on iodine estimations,
e.g., T3 resin uptake and total or free thyroxine (T4) assays are
not affected.
Any test which might be affected by contrast
media should be performed prior to administration of the contrast
medium.
Laboratory Test Findings
In vitro studies with animal
blood showed that many radiopaque contrast agents, including iopamidol,
produced a slight depression of plasma coagulation factors including
prothrombin time, partial thromboplastin time, and fibrinogen, as
well as a slight tendency to cause platelet and/or red blood cell
aggregation (see PRECAUTIONS-General).
Transitory changes may occur in red cell and
leucocyte counts, serum calcium, serum creatinine, serum glutamic
oxaloacetic transaminase (SGOT), and uric acid in urine; transient
albuminuria may occur.
These findings have not been associated
with clinical manifestations.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate carcinogenic potential. No evidence of genetic toxicity was obtained in in vitro tests.
Pregnancy: Teratogenic Effects
Reproduction studies have been performed in rats and rabbits at doses up to 2.7 and 1.4 times the maximum recommended human dose (1.48 gI/kg in a 50 kg individual), respectively, and have revealed no evidence of impaired fertility or harm to the fetus due to iopamidol. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when iopamidol is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in children has been established in pediatric angiocardiography and computed tomography (head and body). Pediatric patients at higher risk of experiencing adverse events during contrast medium administration may include those having asthma, a sensitivity to medication and/or allergens, cyanotic heart disease, congestive heart failure, a serum creatinine greater than 1.5 mg/dL or those less than 12 months of age.
Thyroid function tests indicative of thyroid dysfunction, characterized by hypothyroidism or transient thyroid suppression have been uncommonly reported following iodinated contrast media administration in pediatric patients, including term and preterm neonates; some patients were treated for hypothyroidism. After exposure to iodinated contrast media, individualize thyroid function monitoring in pediatric patients 0 to 3 years of age based on underlying risk factors, especially in term and preterm neonates (see WARNINGS and ADVERSE REACTIONS).
ADVERSE REACTIONS
Adverse reactions following the use of iopamidol
are usually mild to moderate, self-limited, and transient.
In angiocardiography (597 patients), the adverse reactions with
an estimated incidence of one percent or higher are: hot flashes 3.4%;
angina pectoris 3.0%; flushing 1.8%; bradycardia 1.3%; hypotension
1.0%; hives 1.0%.
In a clinical trial with 76 pediatric
patients undergoing angiocardiography, 2 adverse reactions (2.6%)
both remotely attributed to the contrast media were reported. Both
patients were less than 2 years of age, both had cyanotic heart disease
with underlying right ventricular abnormalities and abnormal pulmonary
circulation. In one patient pre-existing cyanosis was transiently
intensified following contrast media administration. In the second
patient pre-existing decreased peripheral perfusion was intensified
for 24 hours following the examination. (See “PRECAUTIONS” Section for information on high risk nature
of these patients.)
Intravascular injection of contrast
media is frequently associated with the sensation of warmth and pain
especially in peripheral arteriography and venography; pain and warmth
are less frequent and less severe with ISOVUE (Iopamidol Injection)
than with diatrizoate meglumine and diatrizoate sodium injection.
The following table of incidence of reactions is based on clinical
studies with ISOVUE in about 2246 patients.
| Adverse Reactions | ||
| Estimated Overall Incidence | ||
| System | > 1% | ≤ 1% |
| Cardiovascular | none | tachycardia hypotension hypertension myocardial ischemia circulatory collapse S-T segment depression bigeminy extrasystoles ventricular fibrillation angina pectoris bradycardia transient ischemic attack thrombophlebitis |
| Nervous | pain (2.8%) burning sensation (1.4%) | vasovagal reaction tingling in arms grimace faintness |
| Digestive | nausea (1.2%) | vomiting anorexia |
| Respiratory | none | throat constriction dyspnea pulmonary edema |
| Skin and Appendages | none | rash urticaria pruritus flushing |
| Body as a Whole | hot flashes (1.5%) | headache fever chills excessive sweating back spasm |
| Special Senses | warmth (1.1%) | taste alterations nasal congestion visual disturbances |
| Urogenital | none | urinary retention |
Regardless of the contrast agent
employed, the overall estimated incidence of serious adverse reactions
is higher with coronary arteriography than with other
procedures. Cardiac decompensation, serious arrhythmias, or myocardial
ischemia or infarction have been reported with ISOVUE and may occur
during coronary arteriographyand left ventriculography.
Following coronary and ventricular injections, certain
electrocardiographic changes (increased QTc, increased R-R, T-wave
amplitude) and certain hemodynamic changes (decreased systolic pressure)
occurred less frequently with ISOVUE (Iopamidol Injection) than with
diatrizoate meglumine and diatrizoate sodium injection; increased
LVEDP occurred less frequently after ventricular iopamidol injections.
In aortography, the risks of procedures also include
injury to the aorta and neighboring organs, pleural puncture, renal
damage including infarction and acute tabular necrosis with oliguria
and anuria, accidental selective filling of the right renal artery
during the translumbar procedure in the presence of pre-existing renal
disease, retroperitoneal hemorrhage from the translumbar approach,
and spinal cord injury and pathology associated with the syndrome
of transverse myelitis.
The following adverse reactions
have been reported for Iopamidol: Cardiovascular: arrhythmia, arterial spasms, flushing, vasodilation, chest pain,
cardiopulmonary arrest; Nervous System: confusion,
paresthesia, dizziness, temporary cortical blindness, temporary amnesia,
convulsions, paralysis, coma; Respiratory:
increased cough, sneezing, asthma, apnea, laryngeal edema, chest tightness,
rhinitis; Skin and Appendages: injection site
pain usually due to extravasation and/or erythematous swelling, pallor,
periorbital edema, facial edema; Urogenital: pain, hematuria; Special Senses: watery
itchy eyes, lacrimation, conjunctivitis; Musculoskeletal: muscle spasm, involuntary leg movement; Body as a whole: tremors, malaise, anaphylactoid reaction (characterized by cardiovascular,
respiratory and cutaneous symptoms), pain; Digestive: severe retching and choking, abdominal cramps. Some of these may
occur as a consequence of the procedure. Other reactions may also
occur with the use of any contrast agent as a consequence of the procedural
hazard; these include hemorrhage or pseudoaneurysms at the puncture
site, brachial plexus palsy following axillary artery injections,
chest pain, myocardial infarction, and transient changes in hepatorenal
chemistry tests. Arterial thrombosis, displacement of arterial plaques,
venous thrombosis, dissection of the coronary vessels and transient
sinus arrest are rare complications.
General Adverse Reactions To Contrast Media
Reactions known to occur with parenteral administration of iodinated ionic contrast agents (see the listing below) are possible with any nonionic agent. Approximately 95 percent of adverse reactions accompanying the use of other water-soluble intravascularly administered contrast agents are mild to moderate in degree. However, life-threatening reactions and fatalities, mostly of cardiovascular origin, have occurred. Reported incidences of death from the administration of other iodinated contrast media range from 6.6 per 1 million (0.00066 percent) to 1 in 10,000 patients (0.01 percent). Most deaths occur during injection or 5 to 10 minutes later, the main feature being cardiac arrest with cardiovascular disease as the main aggravating factor. Isolated reports of hypotensive collapse and shock are found in the literature. The incidence of shock is estimated to be 1 out of 20,000 (0.005 percent) patients.
Adverse reactions to injectable contrast media fall into two categories: chemotoxic reactions and idiosyncratic reactions. Chemotoxic reactions result from the physicochemical properties of the contrast medium, the dose, and the speed of injection. All hemodynamic disturbances and injuries to organs or vessels perfused by the contrast medium are included in this category. Idiosyncratic reactions include all other reactions. They occur more frequently in patients 20 to 40 years old. Idiosyncratic reactions may or may not be dependent on the amount of drug injected, the speed of injection, the mode of injection, and the radiographic procedure. Idiosyncratic reactions are subdivided into minor, intermediate, and severe. The minor reactions are self-limited and of short duration; the severe reactions are life-threatening and treatment is urgent and mandatory.
The reported incidence of adverse reactions to contrast media in patients with a history of allergy is twice that for the general population. Patients with a history of previous reactions to a contrast medium are three times more susceptible than other patients. However, sensitivity to contrast media does not appear to increase with repeated examinations. Most adverse reactions to intravascular contrast agents appear within one to three minutes after the start of injection, but delayed reactions may occur. Delayed reactions, usually involving the skin, may uncommonly occur within 2-3 days (range 1-7 days) after the administration of contrast (see PRECAUTIONS-General). Delayed allergic reactions are more frequent in patients treated with immunostimulants, such as interleukin-2.
In addition to the adverse drug reactions reported for iopamidol, the following additional adverse reactions have been reported with the use of other intravascular contrast agents and are possible with the use of any water-soluble iodinated contrast agent:
Cardiovascular: cerebral hematomas, petechiae; Hematologic: neutropenia; Urogenital: osmotic nephrosis of proximal tubular cells, renal failure; Special Senses: conjunctival chemosis with infection. Endocrine: hyperthyroidism, hypothyroidism; Skin and Subcutaneous Tissue Disorders: Skin necrosis; Reactions range from mild (e.g. rash, erythema, pruritus, uticaria and skin discoloration) to severe: [e.g. Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), cute generalized exanthematous pustulosis (AGEP) and drug reaction with eosinophilia and systemic symptoms (DRESS)].
OVERDOSAGE
Treatment of an overdose of an injectable radiopaque contrast medium is directed toward the support of all vital functions, and prompt institution of symptomatic therapy.
DOSAGE AND ADMINISTRATION
General
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Iopamidol solutions should be used only if clear and within the normal colorless to pale yellow range. Discard any product which shows signs of crystallization or damage to the container-closure system, which includes the glass container, stopper and/or crimp.
It
is desirable that solutions of radiopaque diagnostic agents for intravascular
use be at body temperature when injected. Sterile techniques must
be used with any intravascular injection.
The transferring
of ISOVUE from the ISOVUE Imaging Bulk Package container should be
performed utilizing aseptic technique. The Imaging Bulk Package closure
may be penetrated only one time, with a suitable sterile component
of the automated contrast injection system, contrast management system,
or contrast media transfer set approved or cleared for use with this
Imaging Bulk Package.
Patients should be well
hydrated prior to and following ISOVUE (Iopamidol Injection) administration.
As with all radiopaque contrast agents, only
the lowest dose of ISOVUE necessary to obtain adequate visualization
should be used. A lower dose reduces the possibility of an adverse
reaction. Most procedures do not require use of either a maximum dose
or the highest available concentration of ISOVUE; the combination
of dose and ISOVUE concentration to be used should be carefully individualized,
and factors such as age, body size, size of the vessel and its blood
flow rate, anticipated pathology and degree and extent of opacification
required, structure(s) or area to be examined, disease processes affecting
the patient, and equipment and technique to be employed should be
considered.
Cerebral Arteriography
ISOVUE-300 (Iopamidol Injection, 300 mg iodine/mL) should be used. The usual individual injection by carotid puncture or transfemoral catheterization is 8 to 12 mL, with total multiple doses up to 90 mL.
Peripheral Arteriography
ISOVUE-300 usually provides adequate visualization. For injection into the femoral artery or subclavian artery, 5 to 40 mL may be used; for injection into the aorta for a distal runoff, 25 to 50 mL may be used. Doses up to a total of 250 mL of ISOVUE-300 have been administered during peripheral arteriography.
Peripheral Venography (Phlebography)
ISOVUE-300 should be used. The usual dose is 15 mL to 100 mL per lower extremity. The combined total dose for multiple injections should not exceed 230 mL.
Selective Visceral Arteriography and Aortography
ISOVUE-370 (Iopamidol Injection, 370 mg iodine/mL) should be used. Doses up to 50 mL may be required for injection into the larger vessels such as the aorta or celiac artery; doses up to 10 mL may be required for injection into the renal arteries. Often lower doses will be sufficient. The combined total dose for multiple injections has not exceeded 225 mL.
Pediatric Angiocardiography
ISOVUE-370 should be used. Pediatric angiocardiography may be performed by injection into a large peripheral vein or be direct characterization of the heart. The usual dose range for single injections is provided in the following table:
| Single Injection
Usual Dose Range |
|
| Age | mL |
| <2 years | 10–15 |
| 2–9 years | 15–30 |
| 10–18 years | 20–50 |
The usual recommended dose for cumulative injections is provided in the following table.
| Cumulative Injection
Usual Recommended Dose |
|
| Age | mL |
| <2 years | 40 |
| 2–4 years | 50 |
| 5–9 years | 100 |
| 10–18 years | 125 |
Coronary Arteriography and Ventriculography
ISOVUE-370 should be used. The usual dose for selective coronary artery injections is 2 to 10 mL. The usual dose for ventriculography, or for nonselective opacification of multiple coronary arteries following injection at the aortic root is 25 to 50 mL. The total dose for combined procedures has not exceeded 200 mL. EKG monitoring is essential.
Computed Tomography
CT OF THE HEAD: The suggested dose for
ISOVUE-300 is 100 to 200 mL by intravenous administration. Imaging
may be performed immediately after completion of administration.
CT OF THE BODY: The usual adult dose range for ISOVUE-300 is 100-to
200 mL administered by rapid intravenous infusion or bolus injection.
Equivalent doses of ISOVUE-370 based on organically bound iodine
content may also be used.
The total dose for either CT
procedure should not exceed 60 grams of iodine.
DRUG HANDLING
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Iopamidol solutions should be used only if clear and within the normal colorless to pale yellow range. Discard any product which shows signs of crystallization or damage to the container-closure system, which includes the glass container, stopper and/or crimp.
Directions for Proper Use of the ISOVUE Imaging Bulk Package
The ISOVUE Imaging Bulk Package is used for dispensing multiple single doses of iopamidol injection for multiple patients, using an automated contrast injection system contrast management system, or contrast media transfer set approved or cleared for use with this contrast agent in this Imaging Bulk Package. Preparations of 0.9% Sodium Chloride Injection USP, with a sterile port for an intravenous administration set, are to be used with the ISOVUE Imaging Bulk Package and automated contrast injection systems or contrast management systems approved for use with the ISOVUE Imaging Bulk Package. Please see drug and device labeling for information on devices indicated for use with this Imaging Bulk Package and techniques to help assure safe use.
- The ISOVUE Imaging Bulk Package is to be used only in a room designated for radiological procedures that involve intravascular administration of a contrast agent.
- The transferring of ISOVUE from the Imaging Bulk Package should be performed utilizing aseptic technique. Prior to penetrating the container closure, swab the face of the container stopper with 70% isopropyl alcohol. The container closure may be penetrated only one time with a suitable sterile component of the automated contrast injection system, contrast management system, or contrast media transfer set approved or cleared for use with this Imaging Bulk Package.
- Once the Imaging Bulk Package is punctured, it should not be removed from the work area during the entire period of use, and the bottle should be maintained in an inverted position such that container contents are in continuous contact with the dispensing set.
- A maximum use time of 10 hours from initial closure entry is permitted to complete fluid transfer. Any unused ISOVUE injection must be discarded 10 hours after initial puncture of the Imaging Bulk Package.
- After the container closure is punctured, if the integrity of the Imaging Bulk Package and the delivery system cannot be assured though direct continuous supervision, the Imaging Bulk Package and all associated disposables for the automated contrast injection system, contrast management system, or contrast media transfer set should be discarded.
- Storage temperature of the ISOVUE Imaging Bulk Package container after the closure has been entered should not exceed 25° C (77° F); however, it is desirable that the contents be warmed to body temperature prior to injection.
- If 0.9% Sodium Chloride Injection USP is used, prepare the intravenous port in accordance with the DOSAGE AND ADMINISTRATION section of the approved prescribing information of the product.
- Multiple-dose use of 0.9% Sodium Chloride Injection USP:
- 0.9% Sodium Chloride Injection USP should only be used to deliver multiple doses to multiple patients when used with an automated contrast injection system or contrast management system approved or cleared for multiple-dose use of 0.9% Sodium Chloride Injection.
- The intravenous administration port of the sodium chloride container may be penetrated only one time with a suitable sterile component of the contrast management system approved for use with the ISOVUE Imaging Bulk Package, using aseptic technique. A maximum use time of 10 hours from initial closure entry is permitted to complete the fluid transfer. Any unused sodium chloride must be discarded 10 hours after initial puncture of the 0.9% Sodium Chloride Injection USP container. The container of 0.9% Sodium Chloride Injection USP is to be used only in an area designated for radiological procedures that involve intravascular administration of contrast. All above instructions in c. through e. for the ISOVUE Imaging Bulk Package should be followed for the 0.9% Sodium Chloride Injection USP container. Strap the saline tag provided with the ISOVUE Imaging Bulk Package on the 0.9% Sodium Chloride Injection USP container.
- Single-dose use of 0.9% Sodium Chloride Injection USP: Use in accordance with the manufacturers Prescribing Information.
HOW SUPPLIED
ISOVUE-300 (Iopamidol Injection 61%)
Ten 200 mL Imaging Bulk Packages (NDC 0270-1315-45)
Six 500 mL Imaging Bulk Packages (NDC 0270-1315-95)
ISOVUE-370 (Iopamidol Injection 76%)
Ten 200 mL Imaging
Bulk Packages (NDC 0270-1316-45)
Six 500 mL
Imaging Bulk Packages (NDC 0270-1316-95)