Uses
- for relief of occasional constipation and irregularity
- this product generally produces bowel movement in 15 minutes to 1 hour
Warning
for rectal use only
Do not use
- more than once per day
- for a period of longer than one week unless directed by a doctor
- laxative products when abdominal pain, nausea or vomiting are present unless directed by a doctor
Ask a doctor before use if you have
- noticed a sudden change in bowel habits that persist over a period of two weeks
When using this product you may have abdominal discomfort, faintness, rectal burning and mild cramps
Directions
| Adults and children 12 years of age and older | Children 6 to under 12 years | Children under 6 |
| One suppository once daily | 1/2 suppository once daily | Ask a doctor |
- Detach one suppository from the strip and remove from foil
- Carefully insert well into rectum
- Do not use more than once per day
Other information
- Store at 15°C to 30°C (59°F to 86°F). Do not exceed 30°C (86°F).
- Individually sealed for your protection. Do not use if foil is torn or open.
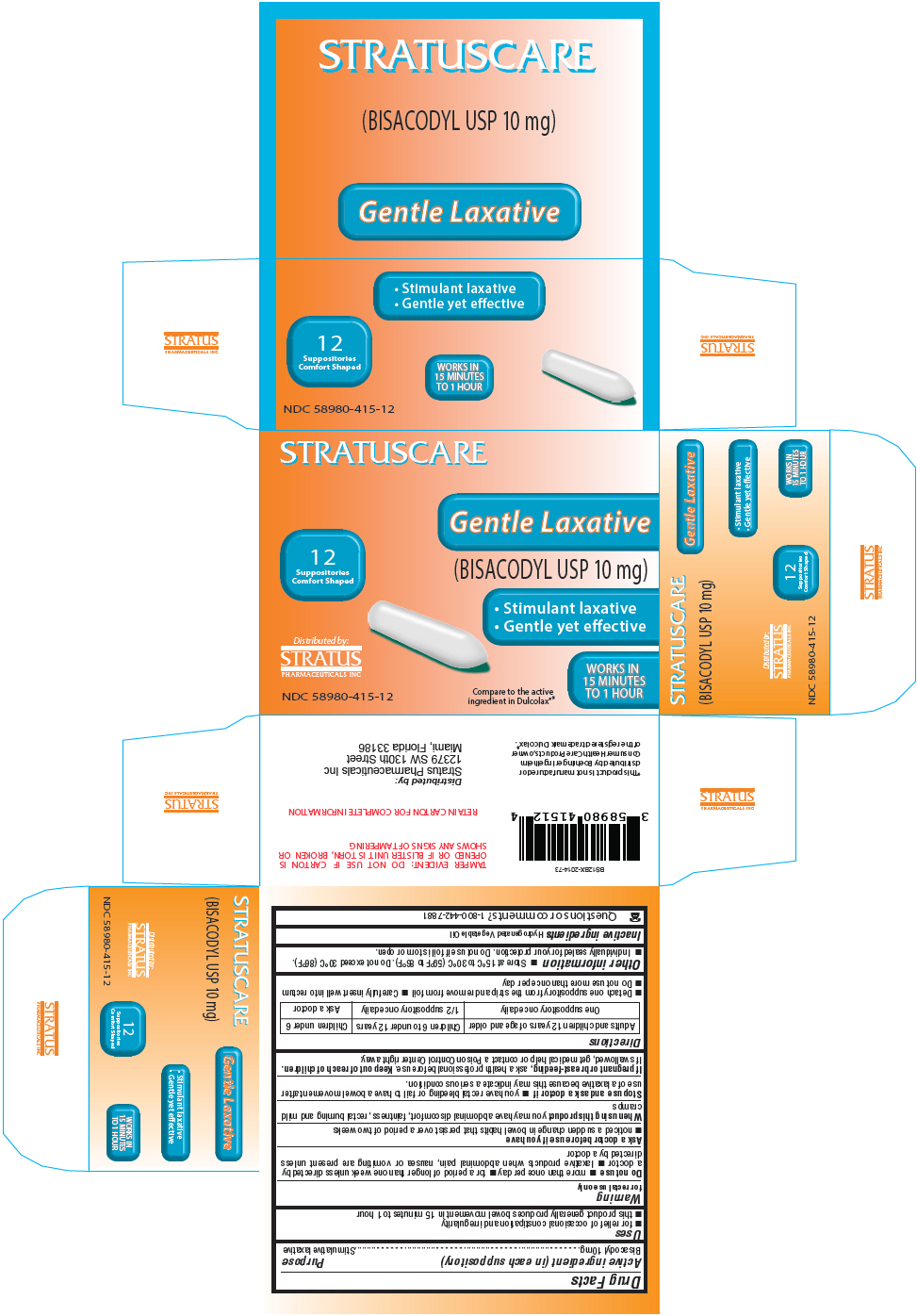
PRINCIPAL DISPLAY PANEL - 10 mg Blister Pack Box
STRATUSCARE
12
Suppositories
Comfort Shaped
Distributed by:
STRATUS
PHARMACEUTICALS INC
NDC 58980-415-12
Gentle Laxative
(BISACODYL USP 10 mg)
- Stimulant laxative
- Gentle yet effective
WORKS IN
15 MINUTES
TO 1 HOUR
Compare to the active
ingredient in Dulcolax®*