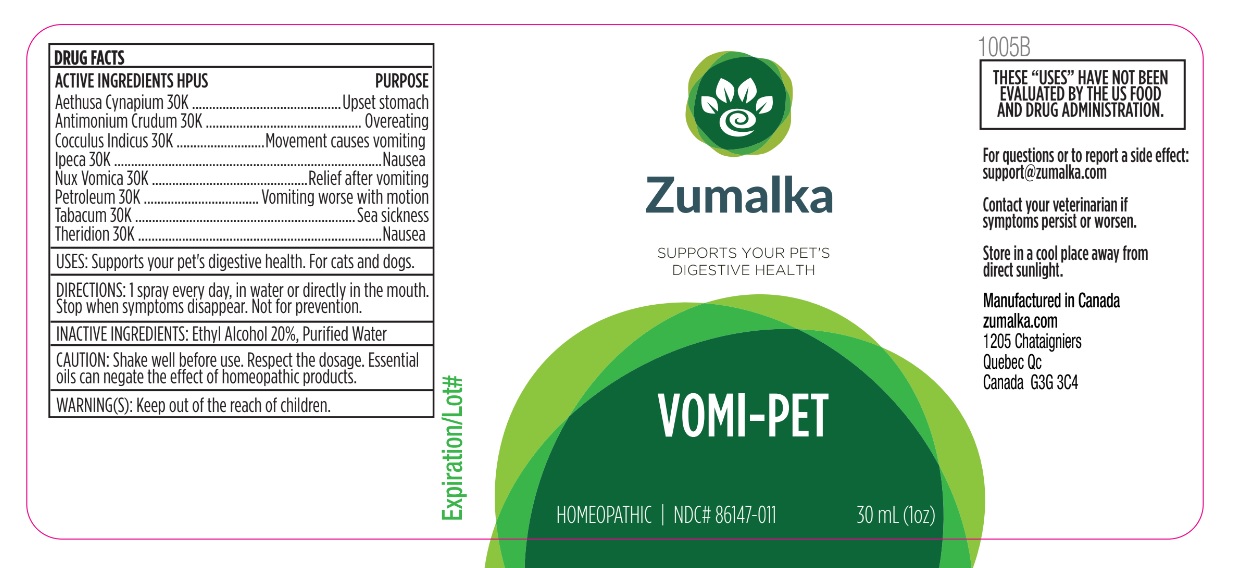
VOMI-PET- aethusa cynapium whole, antimony trisulfide, jateorhiza calumba root, ipecac, strychnos nux-vomica seed, liquid petroleum, tobacco leaf, theridion curassavicum liquid
Groupe Cyrenne Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Active ingredient Purpose
Aethusa cynapium 30k, Upset stomach
Antimonium Crudum 30k, Overeating
Cocculus Indicus 30k, Movement causes vomiting
Ipeca 30k, Nausea
Nux Vomica 30k, Relief after vomiting
Petrolum 30k, Vomiting worse with motion
Tabacum 30k, Sea sickness
Theridion 30k Nausea
Uses
Supports your pet's digestive health. For cats and dogs.
Warnings
Keep out of reach of children.
Direction
1 spray every day, in water or directly in the mouth. Stop when symptoms disappear. Not for prevention.
Inactive ingredient
Ethyl alcohol 20%, purified water
Cautions
Shake well before use. Respect the dosage. Essential oils can negate the effects of homeopathic products. Store in a cool place away from direct sunlight.
Other information
The letters "HPUS" indicate that the active ingredients are in the official Homeopathic Pharmacopoeia of the United States. Homeopathic remedy. Store at room temperature.
Product label
Groupe Cyrenne Inc.
