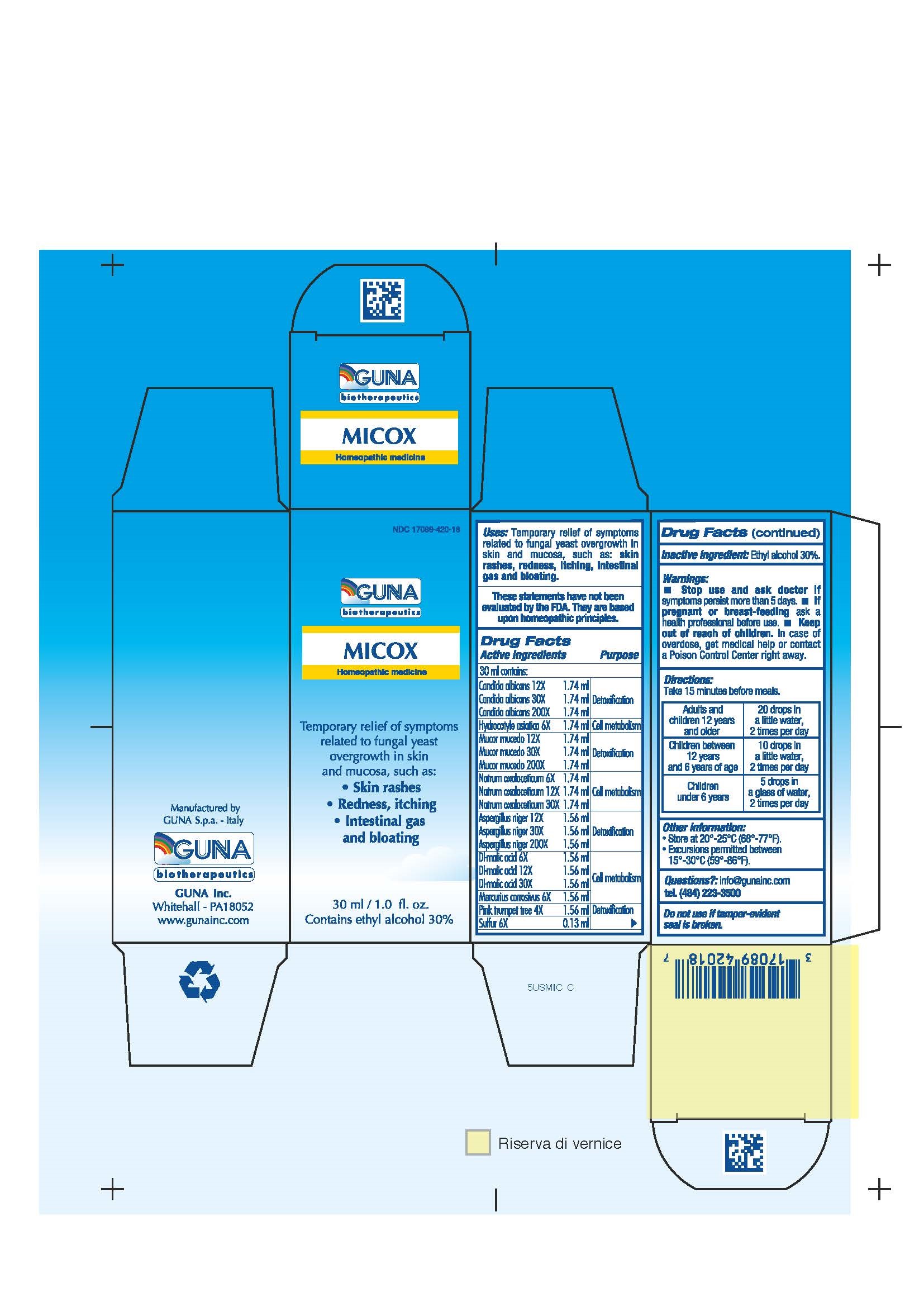
ACTIVE INGREDIENTS/PURPOSE
ASPERGILLUS NIGER 12X, 30X, 200X DETOXIFICATION
CANDIDA ALBICANS 12X, 30X, 200X DETOXIFICATION
DL-MALIC ACID 6X,12X, 30X CELL METABOLISM
HYDROCOTYLE ASIATICA 6X CELL METABOLISM
MERCURIUS CORROSIVUS 6X CELL METABOLISM
MUCOR MUCEDO 12X, 30X, 200X DETOXIFICATION
NATRUM OXALACETICUM 6X,12X, 30X CELL METABOLISM
PINK TRUMPET TREE 4X DETOXIFICATION
SULPHUR 6X DETOXIFICATION
USES
Temporary relief of symptoms related to fungal yeast overgrowth in skin and mucosa, such as:
- Skin rashes
- Redness, itching
- Intestinal gas and bloating
WARNINGS
- Stop use and ask doctor if symptoms persist more than 5 days.
- If pregnant or breast-feeding ask a health professional before use.
- Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
- Contains ethyl alcohol 30%