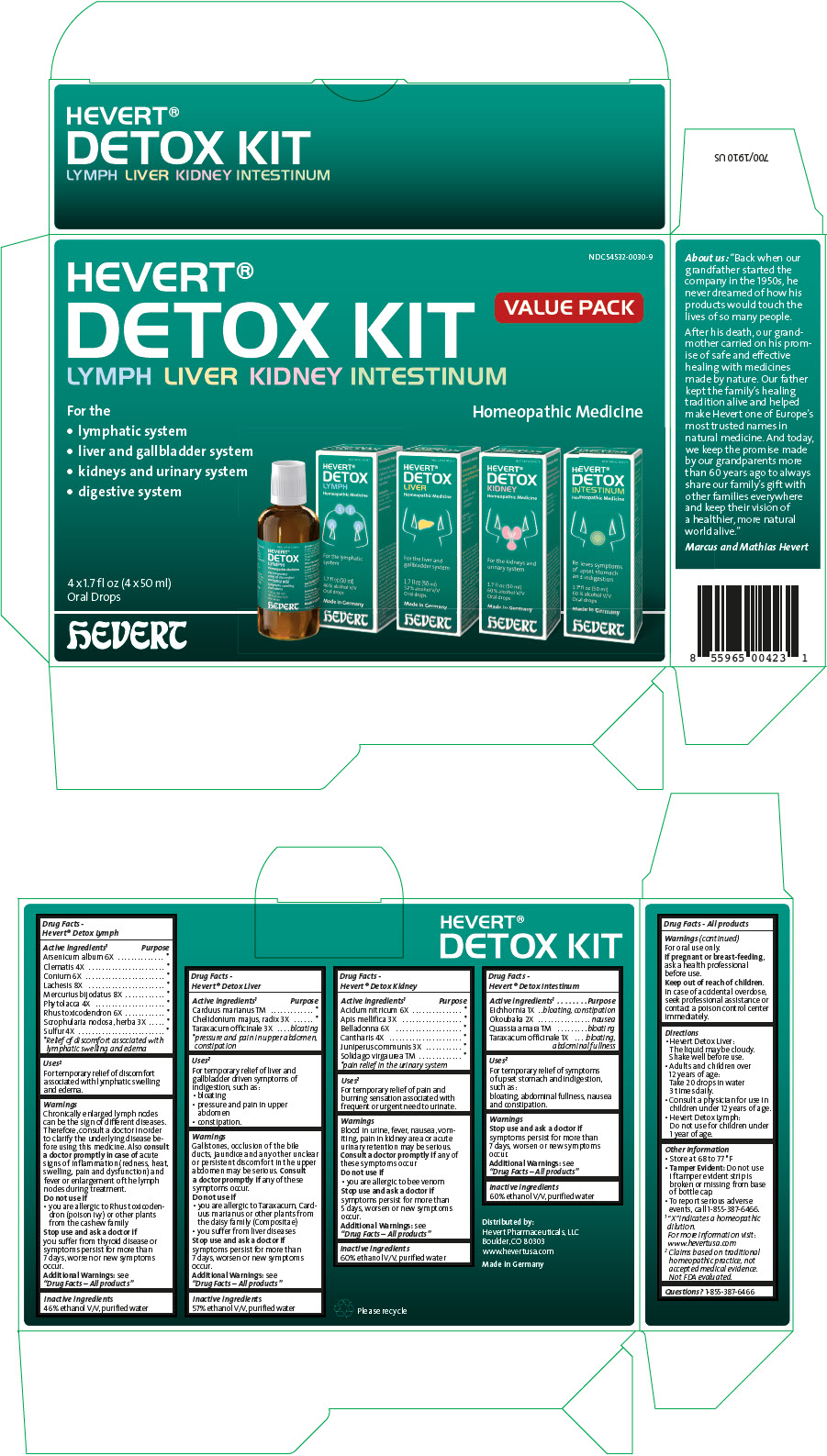
Uses1
For temporary relief of discomfort associated with lymphatic swelling and edema.
- 1
- Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
Warnings
Chronically enlarged lymph nodes can be the sign of different diseases. Therefore, consult a doctor in order to clarify the underlying disease before using this medicine. Also consult a doctor promptly in case of acute signs of inflammation (redness, heat, swelling, pain and dysfunction) and fever or enlargement of the lymph nodes during treatment.
Do not use if
- you are allergic to Rhus toxicodendron (poison ivy) or other plants from the cashew family
Uses2
For temporary relief of liver and gallbladder driven symptoms of indigestion, such as:
- bloating
- pressure and pain in upper abdomen
- constipation.
- 2
- Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
Warnings
Gallstones, occlusion of the bile ducts, jaundice and any other unclear or persistent discomfort in the upper abdomen may be serious. Consult a doctor promptly if any of these symptoms occur.
Uses3
For temporary relief of pain and burning sensation associated with frequent or urgent need to urinate.
- 3
- Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
Warnings
Blood in urine, fever, nausea, vomiting, pain in kidney area or acute urinary retention may be serious. Consult a doctor promptly if any of these symptoms occur
Uses4
For temporary relief of symptoms of upset stomach and indigestion, such as:
bloating, abdominal fullness, nausea and constipation.
- 4
- Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
Directions
- Hevert Detox Liver:
The liquid may be cloudy. Shake well before use. - Adults and children over 12 years of age:
Take 20 drops in water 3 times daily. - Consult a physician for use in children under 12 years of age.
- Hevert Detox Lymph:
Do not use for children under 1 year of age.