To reduce the development of drug-resistant bacteria and maintain the effectiveness of Vancomycin Hydrochloride for Oral Solution and other antibacterial drugs, Vancomycin Hydrochloride for Oral Solution should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
This preparation for the treatment of colitis is for oral use only and is not systemically absorbed. Vancomycin Hydrochloride for Oral Solution must be given orally for treatment of staphylococcal enterocolitis and antibiotic-associated pseudomembranous colitis caused by Clostridium difficile. Orally administered Vancomycin Hydrochloride for Oral Solution is not effective for other types of infection.
Parenteral administration of vancomycin is not effective for treatment of staphylococcal enterocolitis and antibiotic-associated pseudomembranous colitis caused by C. difficile. If parenteral vancomycin therapy is desired, use an intravenous preparation of vancomycin and consult the package insert accompanying that preparation.
DESCRIPTION
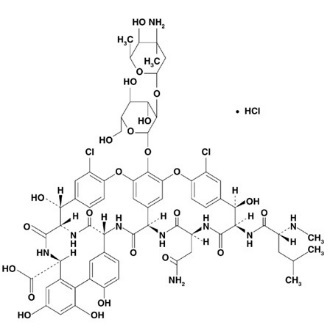
Vancomycin Hydrochloride for Oral Solution USP contains chromatographically purified vancomycin hydrochloride USP, a tricyclic glycopeptide antibiotic derived from Amycolatopsis orientalis (formerly Nocardia orientalis), which has the chemical formula C66H75Cl2N9O24•HCl. The molecular weight of vancomycin hydrochloride is 1,485.73; 500 mg of the base is equivalent to 0.34 mmol.
Vancomycin hydrochloride has the following structural formula:
Vancomycin Hydrochloride for Oral Solution USP is intended for reconstitution with water. Each 5 mL of reconstituted solution contains vancomycin hydrochloride equivalent to 250 mg (0.17 mmol) vancomycin.
Inactive ingredients: citric acid anhydrous, sodium benzoate, sucralose, and mixed berry flavor. Contains no ingredient made from a gluten-containing grain (wheat, barley or rye).
CLINICAL PHARMACOLOGY
Vancomycin is poorly absorbed after oral administration. During multiple dosing of 250 mg every 8 hours for 7 doses, fecal concentrations of vancomycin in volunteers exceeded 100 mg/kg in the majority of samples. No blood concentrations were detected and urinary recovery did not exceed 0.76%. In anephric patients with no inflammatory bowel disease, blood concentrations of vancomycin were barely measurable (0.66 mcg/mL) in 2 of 5 subjects who received 2 g of Vancomycin Hydrochloride for Oral Solution daily for 16 days. No measurable blood concentrations were attained in the other 3 subjects. With doses of 2 g daily, very high concentrations of drug can be found in the feces (>3,100 mg/kg) and very low concentrations (<1 mcg/mL) can be found in the serum of patients with normal renal function who have pseudomembranous colitis. Orally administered vancomycin does not usually enter the systemic circulation even when inflammatory lesions are present. After multiple-dose oral administration of vancomycin, measurable serum concentrations may infrequently occur in patients with active C. difficile-induced pseudomembranous colitis, and, in the presence of renal impairment, the possibility of accumulation exists.
Microbiology
The bactericidal action of vancomycin results primarily from inhibition of cell-wall biosynthesis. In addition, vancomycin alters bacterial-cell-membrane permeability and RNA synthesis.
NOTE: The oral form of vancomycin is effective only for the infections noted in the INDICATIONS AND USAGE section. The oral form is not effective for any other type of infection.
Vancomycin has been shown to be active against most strains of the following microorganisms in clinical infections as described in the INDICATIONS AND USAGE section.
Aerobic gram-positive microorganisms
Staphylococcus aureus (including methicillin-resistant strains) associated with enterocolitis
Aerobic gram-positive microorganisms
Clostridium difficile antibiotic-associated pseudomembranous colitis
INDICATIONS AND USAGE
Vancomycin Hydrochloride for Oral Solution is administered orally for treatment of enterocolitis caused by Staphylococcus aureus (including methicillin-resistant strains) and antibiotic-associated pseudomembranous colitis caused by C. difficile. Parenteral administration of vancomycin is not effective for the above indications; therefore, Vancomycin Hydrochloride for Oral Solution must be given orally for these infections. Orally administered Vancomycin Hydrochloride for Oral Solution is not effective for other types of infection.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Vancomycin Hydrochloride for Oral Solution and other antibacterial drugs, Vancomycin Hydrochloride for Oral Solution should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
CONTRAINDICATIONS
Vancomycin Hydrochloride for Oral Solution is contraindicated in patients with known hypersensitivity to vancomycin.
WARNINGS
Nephrotoxicity
Nephrotoxicity (e.g., reports of renal failure, renal impairment, blood creatinine increased) has occurred following oral vancomycin hydrochloride therapy in randomized controlled clinical trials, and can occur either during or after completion of therapy. The risk of nephrotoxicity is increased in patients over 65 years of age.
In patients over 65 years of age, including those with normal renal function prior to treatment, renal function should be monitored during and following treatment with vancomycin oral solution to detect potential vancomycin induced nephrotoxicity.
Ototoxicity
Ototoxicity has occurred in patients receiving vancomycin. It may be transient or permanent. It has been reported mostly in patients who have been given excessive intravenous doses, who have an underlying hearing loss, or who are receiving concomitant therapy with another ototoxic agent, such as an aminoglycoside. Serial tests of auditory function may be helpful in order to minimize the risk of ototoxicity.
Severe Dermatologic Reactions
Severe dermatologic reactions such as toxic epidermal necrolysis (TEN), Stevens-Johnson syndrome (SJS), drug reaction with eosinophilia and systemic symptoms (DRESS), acute generalized exanthematous pustulosis (AGEP), and linear IgA bullous dermatosis (LABD) have been reported in association with the use of vancomycin. Cutaneous signs or symptoms reported include skin rashes, mucosal lesions, and blisters.
Discontinue Vancomycin Hydrochloride for Oral Solution at the first appearance of signs and symptoms of TEN, SJS, DRESS, AGEP, or LABD.
Clostridium Difficile Associated Diarrhea (CDAD)
Significant systemic absorption has been reported in some patients (e.g., patients with renal insufficiency and/or colitis) who have taken multiple oral doses of vancomycin hydrochloride for C. difficile-associated diarrhea. In these patients, serum vancomycin concentrations reached therapeutic levels for the treatment of systemic infections. Some patients with inflammatory disorders of the intestinal mucosa also may have significant systemic absorption of vancomycin. These patients may be at risk for the development of adverse reactions associated with higher doses of vancomycin oral solution; therefore, monitoring of serum concentrations of vancomycin may be appropriate in some instances, e.g., in patients with renal insufficiency and/or colitis or in those receiving concomitant therapy with an aminoglycoside antibacterial drug.
PRECAUTIONS
Use of vancomycin may result in the overgrowth of non-susceptible bacteria. If superinfection occurs during therapy, appropriate measures should be taken.
Prescribing vancomycin in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to the patient and increases the risk of the development of drug resistant bacteria.
Hemorrhagic occlusive retinal vasculitis, including permanent loss of vision, occurred in patients receiving intracameral or intravitreal administration of vancomycin during or after cataract surgery. The safety and efficacy of vancomycin administered by the intracameral or intravitreal route have not been established by adequate and well-controlled studies. Vancomycin is not indicated for prophylaxis of endophthalmitis.
Pregnancy
Animal reproduction studies have not been conducted with vancomycin. It is not known whether vancomycin can affect reproduction capacity. In a controlled clinical study, the potential ototoxic and nephrotoxic effects of vancomycin on infants were evaluated when the drug was administered intravenously to pregnant women for serious staphylococcal infections complicating intravenous drug abuse. Vancomycin was found in cord blood. No sensorineural hearing loss or nephrotoxicity attributable to vancomycin was noted. One infant whose mother received vancomycin in the third trimester experienced conductive hearing loss that was not attributed to the administration of vancomycin. Because the number of patients treated in this study was limited and vancomycin was administered only in the second and third trimesters, it is not known whether vancomycin causes fetal harm. Vancomycin Hydrochloride for Oral Solution should be given to a pregnant woman only if clearly needed.
Nursing Mothers
Vancomycin is excreted in human milk based on information obtained with the intravenous administration of vancomycin. However, systemic absorption of vancomycin is very low following oral administration of Vancomycin Hydrochloride for Oral Solution (see CLINICAL PHARMACOLOGY). It is not known whether oral vancomycin is excreted in human milk, as no studies of vancomycin concentration in human milk after oral administration have been done. Caution should be exercised when Vancomycin Hydrochloride for Oral Solution is administered to a nursing woman. Because of the potential for adverse events, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Geriatric Use
In clinical trials, 54% of vancomycin hydrochloride-treated subjects were > 65 years of age. Of these, 40% were between the ages of > 65 and 75, and 60% were > 75 years of age.
Clinical studies with vancomycin hydrochloride in C. difficile-associated diarrhea have demonstrated that geriatric subjects are at increased risk of developing nephrotoxicity following treatment with oral vancomycin hydrochloride, which may occur during or after completion of therapy. In patients over 65 years of age, including those with normal renal function prior to treatment, renal function should be monitored during and following treatment with vancomycin hydrochloride to detect potential vancomycin induced nephrotoxicity.
Patients over 65 years of age may take longer to respond to therapy compared to patients 65 years of age and younger. Clinicians should be aware of the importance of appropriate duration of vancomycin hydrochloride treatment in patients over 65 years of age and not discontinue or switch to alternative treatment prematurely.
Information for Patients
Severe Dermatologic Reactions
Advise patients about the signs and symptoms of serious skin manifestations. Instruct patients to stop taking Vancocin immediately and promptly seek medical attention at the first signs or symptoms of skin rash, mucosal lesions and blisters (see WARNINGS).
Patients should be counseled that antibacterial drugs including Vancomycin Hydrochloride for Oral Solution should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Vancomycin Hydrochloride for Oral Solution is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Vancomycin Hydrochloride for Oral Solution or other antibacterial drugs in the future.
ADVERSE REACTIONS
Nephrotoxicity
Nephrotoxicity (e.g., reports of renal failure, renal impairment, blood creatinine increased) occurred in 5% of subjects treated with vancomycin hydrochloride. Nephrotoxicity following vancomycin hydrochloride typically first occurred within one week after completion of treatment (median day of onset was Day 16). Nephrotoxicity following vancomycin hydrochloride occurred in 6% of subjects over 65 years of age and 3% of subjects 65 years of age and younger. Nephrotoxicity can also occur during oral vancomycin administration.
The incidences of hypokalemia, urinary tract infection, peripheral edema, insomnia, constipation, anemia, depression, vomiting, and hypotension were higher among subjects over 65 years of age than in subjects 65 years of age and younger.
Discontinuation of study drug due to adverse events occurred in 7% of subjects treated with vancomycin hydrochloride. The most common adverse events leading to discontinuation of vancomycin hydrochloride were C. difficile colitis (< 1%), nausea (< 1%), and vomiting (< 1%).
Ototoxicity
Cases of hearing loss associated with intravenously administered vancomycin have been reported. Most of these patients had kidney dysfunction or a preexisting hearing loss or were receiving concomitant treatment with an ototoxic drug. Vertigo, dizziness, and tinnitus have been reported rarely.
Hematopoietic
Reversible neutropenia, usually starting 1 week or more after onset of intravenous therapy with vancomycin or after a total dosage of more than 25 g, has been reported for several dozen patients. Neutropenia appears to be promptly reversible when vancomycin is discontinued. Thrombocytopenia has rarely been reported.
Miscellaneous
Patients have been reported to have had anaphylaxis, drug fever, nausea, chills, eosinophilia, rashes including exfoliative dermatitis, Stevens-Johnson syndrome (see WARNINGS, Severe Dermatologic Reactions), and vasculitis in association with the administration of vancomycin.
A condition has been reported that is similar to the IV-induced syndrome with symptoms consistent with anaphylactoid reactions, including hypotension, wheezing, dyspnea, urticaria, pruritus, flushing of the upper body (“Red Man Syndrome”), pain and muscle spasm of the chest and back. These reactions usually resolve within 20 minutes but may persist for several hours.
Post Marketing Reports
Skin and Subcutaneous Tissue Disorders
Severe dermatologic reactions such as toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), acute generalized exanthematous pustulosis (AGEP), and linear IgA bullous dermatosis (LABD) (see WARNINGS, Severe Dermatologic Reactions).
OVERDOSAGE
Supportive care is advised, with maintenance of glomerular filtration. Vancomycin is poorly removed by dialysis. Hemofiltration and hemoperfusion with polysulfone resin have been reported to result in increased vancomycin clearance.
For current information on the management of overdosage, contact the National Poison Control Center at 1-800-222-1222 or www.poison.org.
DOSAGE AND ADMINISTRATION
Adults
Vancomycin Hydrochloride for Oral Solution is used in treating antibiotic-associated pseudomembranous colitis caused by C. difficile and staphylococcal enterocolitis. Vancomycin Hydrochloride for Oral Solution is not effective by the oral route for other types of infections. The usual adult total daily dosage is 500 mg to 2 g administered orally in 3 or 4 divided doses for 7 to 10 days.
PREPARATION AND STABILITY
Mix the contents of the bottle with water as directed below. When reconstituted, each 5 mL contains approximately 250 mg of vancomycin. These mixtures may be kept for two weeks in a refrigerator without significant loss of potency.
Directions for mixing Vancomycin Hydrochloride for Oral Solution USP:
80 mL – Slowly add 80 mL water and shake vigorously.
150 mL – Slowly add 150 mL water and shake vigorously.
300 mL – Slowly add 300 mL water and shake vigorously.
The appropriate oral solution dose may be diluted in 1 oz of water and given to the patient to drink. The diluted material may be administered via nasogastric tube.
HOW SUPPLIED
Vancomycin Hydrochloride for Oral Solution USP equivalent to 250 mg per 5 mL vancomycin is available as:
80 mL bottle (4 g*) NDC 63629-1970-1
* Equivalent to vancomycin
Store at refrigerated conditions, 2° to 8°C (36° to 46°F).
After mixing, refrigerate and use within two weeks. Shake well before using. Keep tightly closed.
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504