PHENAZO- phenozapyridine hydrochloride tablet
Contract Pharmacal Corp.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
0064
DESCRIPTION
Phenazopyridine Hydrochloride is light or dark red to dark violet, odorless, slightly bitter, crystalline powder. It has a specific local analgesic effect in the urinary tract, promptly relieving burning and pain. It has the following structural formula.
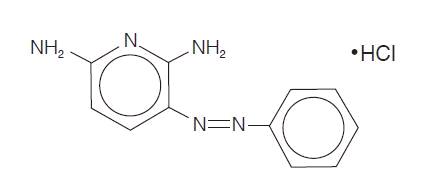
Phenazopyridine HCl tablets contain the following
INACTIVE INGREDIENT
Lactose, Magnesium Silicate, Magnesium Stearate, Microcrystalline Cellulose, Pharmaceutical Glaze and Sodium Starch Glycolate.
CLINICAL PHARMACOLOGY
Phenazopyridine HCl is excreted in the urine where it exerts a topical analgesic effect on the mucosa of the urinary tract. This action helps to relieve pain, burning, urgency and frequency. The precise mechanism of action is not known.
The pharmacokinetic properties of Phenazopyridine HCl have not been determined. Phenazopyridine HCl is rapidly excreted by the kidneys, with as much as 65% of an oral dose being excreted unchanged in the urine.
INDICATIONS AND USAGE
Phenazopyridine HCl is indicated for the symptomatic relief of pain, burning, urgency,
frequency, and other discomforts arising from irritation of the lower urinary tract mucosa caused by infection, trauma, surgery, endoscopic procedures, or the passage of sounds or catheters. The use of Phenazopyridine HCl for relief of symptoms should not delay definitive diagnosis and treatment of causative conditions. Because it provides only symptomatic relief, prompt appropriate treatment of the cause of pain must be instituted and Phenazopyridine HCl should be discontinued when symptoms are controlled.
The analgesic action may reduce or eliminate the need for systemic analgesics or narcotics. It is, however, compatible with antibacterial therapy and can help to relieve pain and discomfort during the interval before antibacterial therapy controls the infection. Treatment of a urinary tract infection with Phenazopyridine HCl should not exceed 2 days because there is a lack of evidence that the combined administration of Phenazopyridine HCl and an antibacterial provides greater benefit than administration of the antibacterial alone after two days. (See DOSAGE AND ADMINISTRATION section.)
CONTRAINDICATIONS
Phenazopyridine HCl should not be used in patients who have previously exhibited hypersensitivity to it. The use of Phenazopyridine HCl is contraindicated in patients with renal insufficiency.
ADVERSE REACTIONS
Headache, rash, pruritus and occasional gastrointestinal disturbance. An anaphylactoid-like reaction has been described. Methemoglobinemia, hemolytic anemia, renal and hepatic toxicity have been described, usually at overdose levels (see Overdosage Section).
PRECAUTIONS
General
A yellowish tinge of the skin or sclera may indicate accumulation due to impaired renal excretion and the need to discontinue therapy. The decline in renal function associated with advanced age should be kept in mind.
NOTE: Patients should be informed that Phenazopyridine HCl produces a reddish-orange discoloration of the urine and may stain fabric. Staining of contact lenses has been reported.
Laboratory Test Interaction
Due to its property as an azo dye, Phenazopyridine HCl may interfere with urinalysis based on spectrometry or color reactions.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term administration of Phenazopyridine HCl has induced neoplasia in rats (large intestine) and mice (liver); although no association between Phenazopyridine HCl and human neoplasia has been reported, adequate epidemiological studies along these lines have not been conducted.
Pregnancy Category B
Reproduction studies have been performed in rats at doses up to 50 mg/kg/day and have revealed no evidence of impaired fertility or harm to the fetus due to Phenazopyridine HCl. There are however, no adequate and well controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
DOSAGE AND ADMINISTRATION
95 mg. Tablets: Average adult dosage is two tablets 3 times a day after meals.
When used concomitantly with an antibacterial agent for the treatment of a urinary tract infection, the administration of Phenazopyridine HCl should not exceed 2 days.
OVERDOSAGE
Exceeding the recommended dose in patients with good renal function or administering the usual dose to patients with impaired renal function (common in elderly patients) may lead to increased serum levels and toxic reactions. Methemoglobinemia generally follows a massive, acute overdose. Methylene blue, 1 to 2 mg/kg/body weight intravenously or ascorbic acid 100 to 200 mg. given orally should cause prompt reduction of the methemoglobinemia and disappearance of the cyanosis which is an aid in diagnosis. Oxidative Heinz Body Hemolytic Anemia may also occur, and "bite cells" (degmacytes) may be present in a chronic overdosage situation. Red blood cell G-6-PD deficiency may predispose to hemolysis. Renal and hepatic impairment and occasional failure, usually due to hypersensitivity, may also occur.
WARNINGS
Ask a doctor before use if you have
- kidney disease
- allergies to foods, preservatives or dyes
- had a hypersensitive reaction to Phenazopyridine
Caution: Do not use this product if you have Glucose-6-Phosphate Dehydrogenase (G6PD) deficiency unless approved by your physician.
When using this product
- stomach upset may occur, taking this product with or after meals may reducer stomach upset
- your urine will become reddish-orange in color. This is not harmful, but care should be taken to avoid staining clothing or other items.
Stop use and ask a doctor if
- your symptoms last more than two days
- you suspect you are having an adverse reaction to the medication.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children
In case of overdose, get medical help or a Poison Control Center right away.
| PHENAZO
phenozapyridine hydrochloride tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Contract Pharmacal Corp. (057795122) |
| Registrant - Contract Pharmacal Corp. (057795122) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Contract Pharmacal Corp | 968334974 | manufacture(10267-0064) | |
