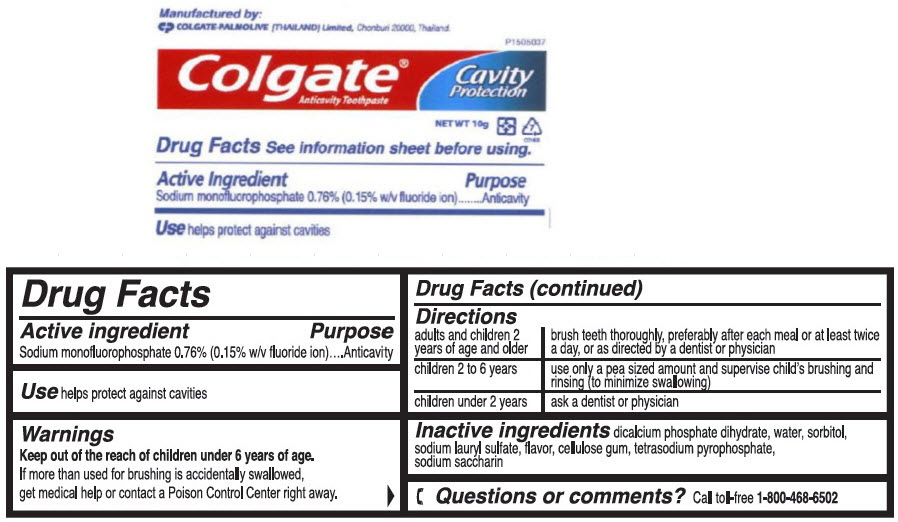
Directions
| adults and children 2 years of age and older | brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or physician |
| children 2 to 6 years | use only a pea sized amount and supervise child's brushing and rinsing (to minimize swallowing) |
| children under 2 years | ask a dentist or physician |
Inactive ingredients
dicalcium phosphate dihydrate, water, sorbitol, sodium lauryl sulfate, flavor, cellulose gum, tetrasodium pyrophosphate, sodium saccharin
Directions
Rub dime sized amount between hands until dry.
Children under 6 years of age should be supervised when using this product.
Inactive Ingredients
Glycerin, Methylisothiazolinone, Propylene Glycol, Polyquaternium-37, Triethanolamine, Water
PRINCIPAL DISPLAY PANEL - 10 g Tube Label
P1505037
Colgate®
Anticavity Toothpaste
Cavity
Protection
NET WT 10g
7
OTHER
PRINCIPAL DISPLAY PANEL - 53 mL Bottle Label
ASP
MEDICAL
Instant Hand Antiseptic
Alcohol & Fragrance Free
1.9 FL OZ / 53 mL
Distributed by:
ASP Global, LLC
7800 Third Flag Parkway,
Austell, GA 30168, USA
Rev 02

PRINCIPAL DISPLAY PANEL - Kit Label
We strive to provide a quiet environment for you
to rest in during your stay. This Welcome Packet
contains some items that are helpful in reducing
hospital noise.
The best healing happens in a quiet environment.
You can help by:
- Using a headset when watching TV
- Keeping cell phones on vibrate
- Speaking softly
- Asking family and friends to visit two at a time
We are honored to care for you.
Contents/Origins:
1.9 fl. oz. Alcohol and Fragrance Free Hand Sanitizer,
0.15 oz. net wt. Lip Balm,
Toothbrush, Sleep Mask, Ear Plugs, Notebook, Pen and
Kit Case: Made in China
Toothpaste: Made in Thailand
Lot #: XMMDDFC
Exp: YYYY-MM-DD
Distributed by:
ASP Global, LLC
7800 Third Flag Parkway, Austell, GA 30168
REV 01
THE
University of Vermont
MEDICAL CENTER
Item #: UNVMC01
Description: AMENITY KIT, UVMC,
GREEN, ZIPPERED
PO #:
Lot #: XMMDDFC
Exp: YYYY-MM-DD
Qty: 30 KIT/CS
Carton #: XXX of XXXX
Net Wt.: XX KG
Gross Wt.: XX KG
Cubic Dimensions: 57.0 x 17.0 x 27.0 CM
Assembled in China
Components Made in China:
- Alcohol and Fragrance Free Hand Sanitizer
- Lip Balm
- Toothbrush
- Sleep Mask
- Ear Plugs
- Notebook
- Pen
- Kit Case
Component Made in Thailand:
- Toothpaste
THE
University of Vermont
MEDICAL CENTER
