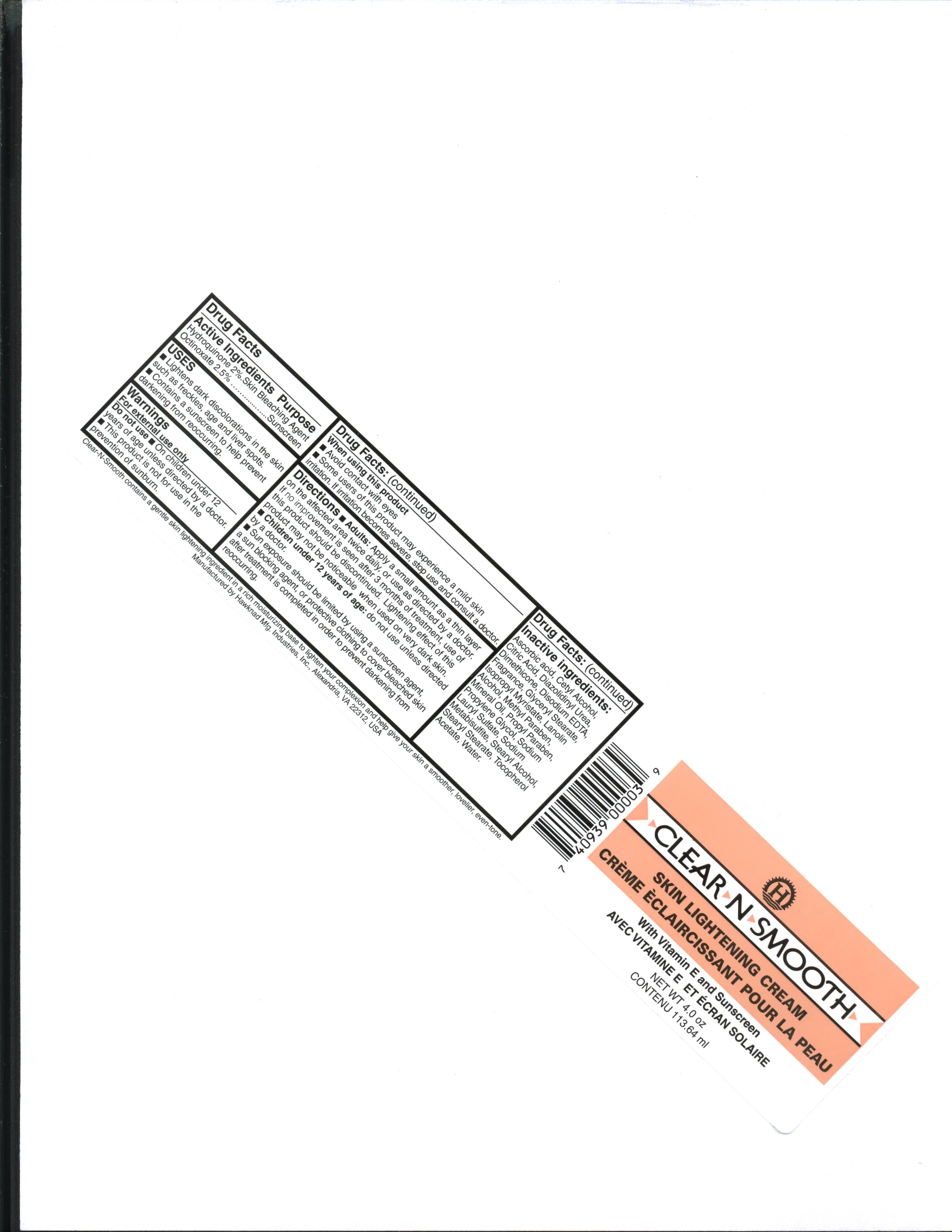
Indications for Use
- Lightens dark colorations in the skin such as freckles, age, and liver spots
- Contains a sunscreen to help prevent darkening from re-occurring.
Keep Out of Reach of Children
Children under 12 years of age: do not use unless directed by a doctor.
Warnings
Do not use on children under 12 years of age unless directed by a doctor.
This product is not for use for the prevention of sunburn.
When using this product avoid contact with eyes.
Some users of this product may experience a mild skin irritation. If irritation becomes severe, stop use and consult a doctor.
Instructions for Use
Apply a small amount as a thin layer on the affected area twice daily, or use as directed by a doctor. If no improvement is seen after 3 months of treatment, use of this product should be discontinued. Lightening effect of this product may not be noticeable when used on very dark skin. Sun exposure should be limited by using a sunscreen agent, a protective clothing to cover bleached skin after treatment is completed in order to prevent darkening from re-occurring.
Inactive Ingredients
ascorbic acid, cetyl alcohol, citric acid, diazolidinyl urea, dimethicone, disodium EDTA, Fragrance, glyceryl stearate, isopropyl myristate, lanolin alcohol, methyl paraben, propyl paraben, mineral oil, propylene glycol, sodium lauryl sulfate, sodium metabisulfite, stearyl alcohol, stearyl stearate, tocopherol acetate, glabridin, water.