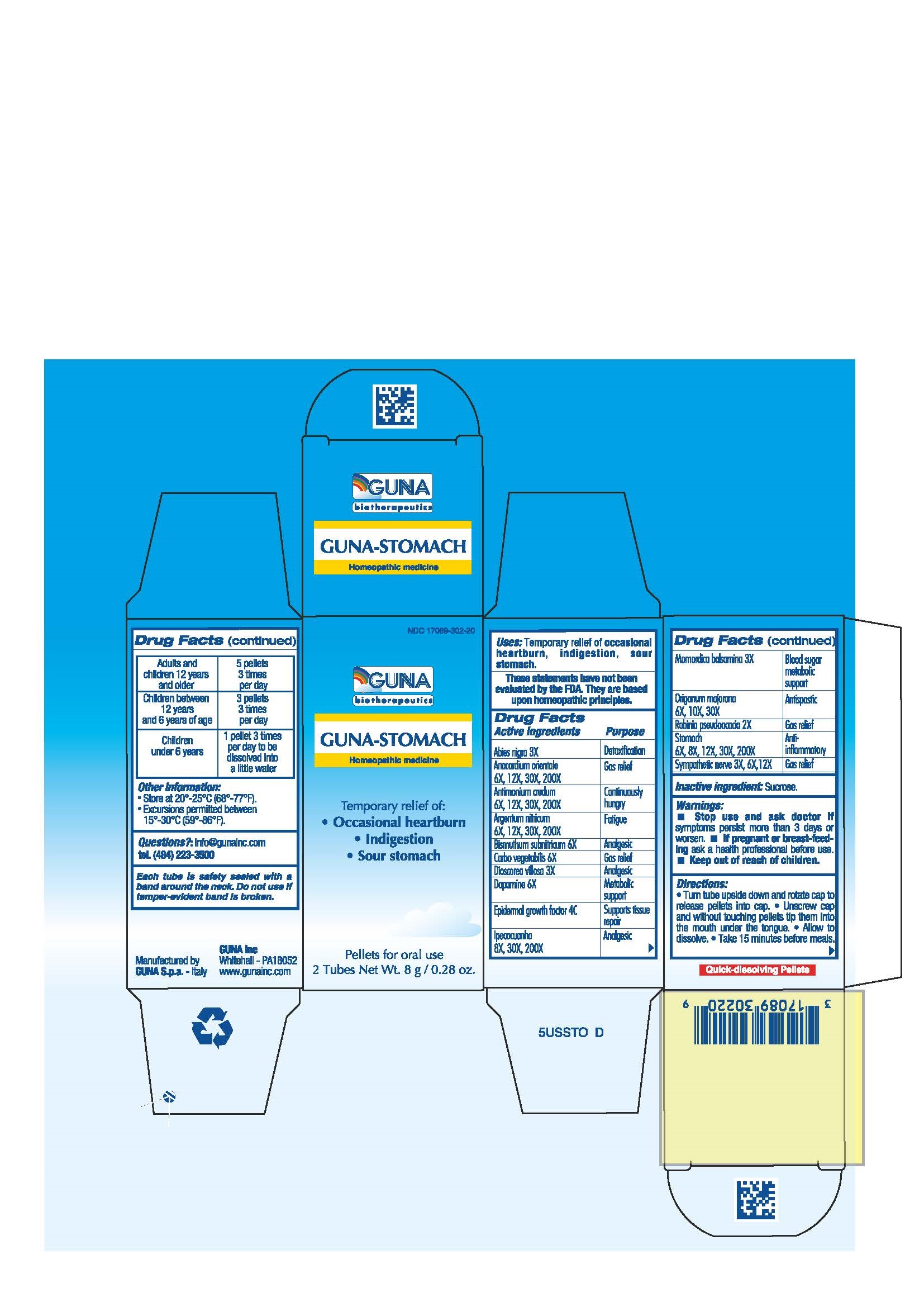
ACTIVE INGREDIENTS/PURPOSE
ABIES NIGRA 3X DETOXIFICATION
ANACARDIUM ORIENTALE 6X, 12X, 30X, 200X GAS RELIEF
ANTIMONIUM CRUDUM 6X, 12X, 30X, 200X CONTINUOUSLY HUNGRY
ARGENTUM NITRICUM 6X, 12X, 30X, 200X FATIGUE
BISMUTHUM SUBNITRICUM 6X ANALGESIC
CARBO VEGETALIS 6X GAS RELIEF
DIOSCOREA VILLOSA 3X ANALGESIC
DOPAMINE 6X METABOLIC SUPPORT
EPIDERMAL GROWTH FACTOR 4C SUPPORT TISSUE REPAIR
IPECACUANHA 8X, 30X, 200X ANALGESIC
MOMORDICA BALSAMINA 3X BLOOD SUGAR METABOLIC SUPPORT
ORIGANUM MAJORANA 6X, 10X, 30X ANTISPASTIC
ROBINIA PSEUDOACACIA 2X GAS RELIEF
STOMACH 6X, 8X, 12X, 30X, 200X ANTI-INFLAMMATORY
SYMPATHETIC NERVE 3X, 6X, 12X GAS RELIEF
WARNINGS
- Stop use and ask doctor if symptoms persist more than 3 days or worsen.
- If pregnant or breast-feeding ask a health professional before use.
- Keep out of reach of children.
DIRECTIONS
- Turn tube upside down and rotate cap to release pellets into cap.
- Unscrew cap and without touching pellets tip them into the mouth under the tongue.
- Allow to dissolve
- Take 15 minutes before meals.