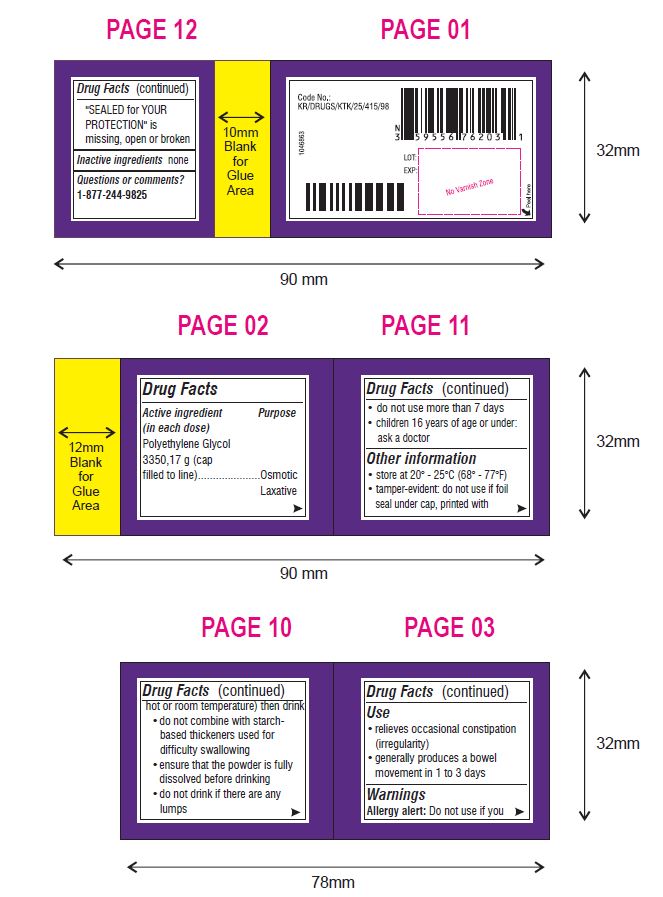
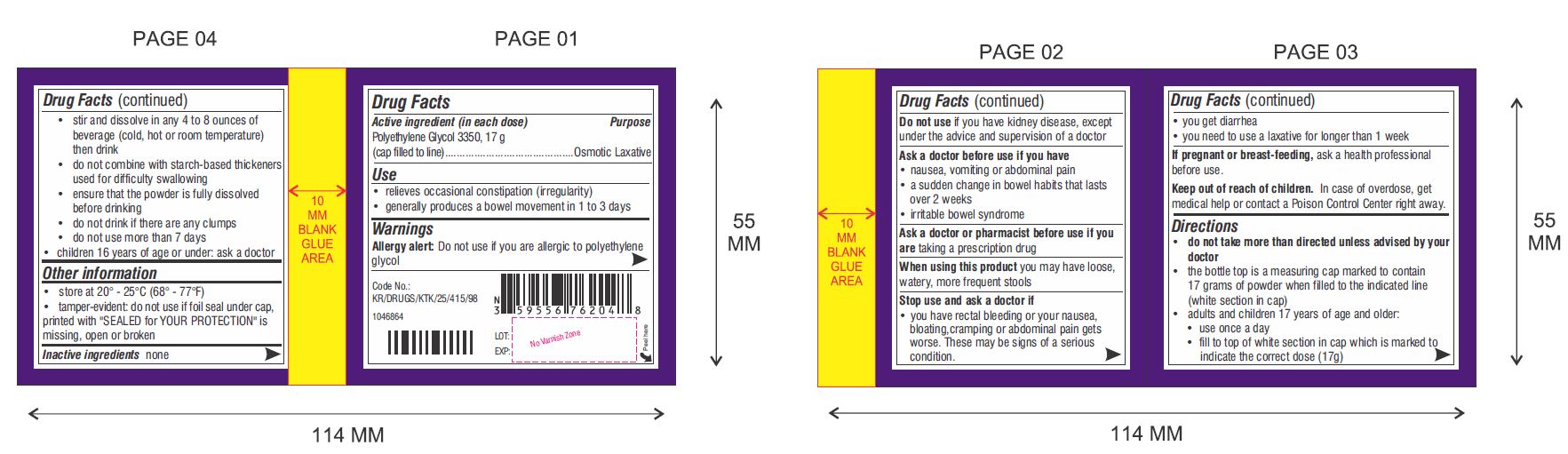
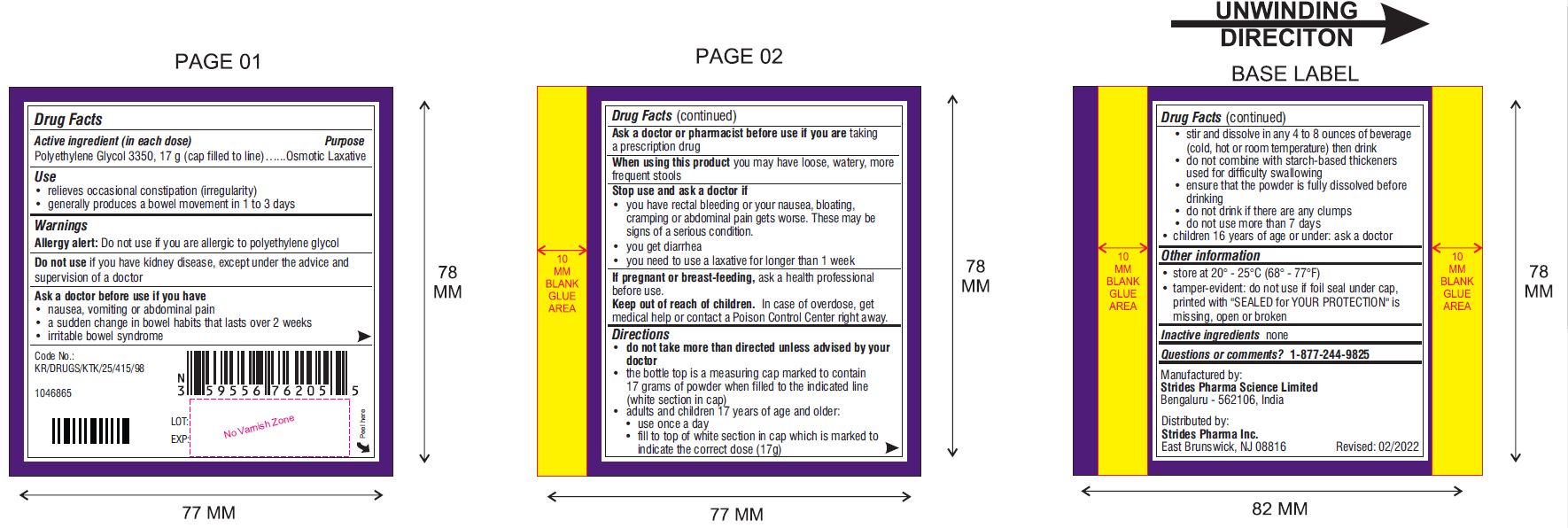
Use
- relieves occasional constipation (irregularity)
- generally produces a bowel movement in 1 to 3 days
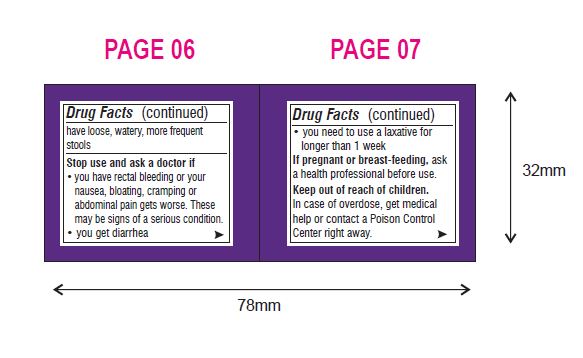
Warnings
Ask a doctor before use if you have
- nausea, vomiting or abdominal pain
- a sudden change in bowel habits that lasts over 2 weeks
- irritable bowel syndrome
Directions (Can Only)
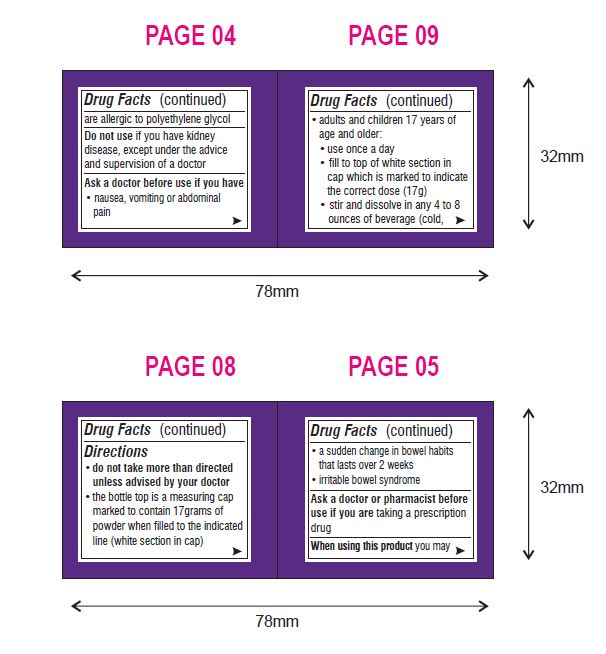
- do not take more than directed unless advised by your doctor
- the bottle top is a measuring cap marked to contain 17 grams of powder when filled to the indicated line (white section in cap)
- adults and children 17 years of age and older:
- use once a day
- fill to top of white section in cap which is marked to indicate the correct dose (17 g)
- stir and dissolve in any 4 to 8 ounces of beverage (cold, hot or room temperature) then drink
- do not combine with starch-based thickeners used for difficulty swallowing
- ensure that the powder is fully dissolved before drinking
- do not drink if there are any clumps
- do not use more than 7 days
- children 16 years of age or under: ask a doctor
Directions (Packet Only)
- do not take more than directed unless advised by your doctor
- adults and children 17 years of age and older:
- use once a day
- stir and dissolve one packet of powder (17 g) in any 4 to 8 ounces of beverage (cold, hot or room temperature) then drink
- do not combine with starch-based thickeners used for difficulty swallowing
- ensure that the powder is fully dissolved before drinking
- do not drink if there are any clumps
- do not use more than 7 days
- children 16 years of age or under: ask a doctor
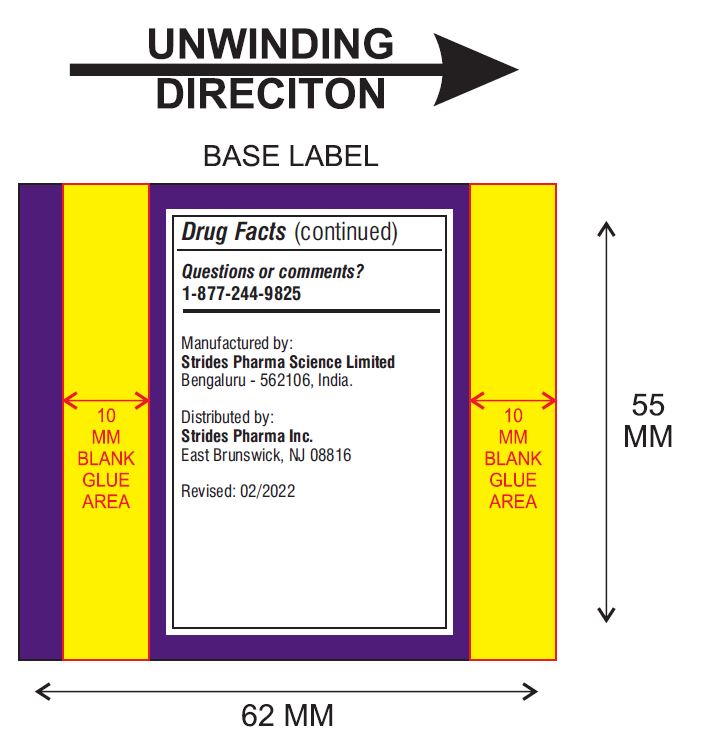
Other Information
- store at 20° - 25°C (68° - 77°F)
- tamper-evident: do not use if foil seal under cap, printed with "SEALED for YOUR PROTECTION" is missing, open or broken
Questions or comments?
Polyethylene Glycol 3350 powder for solution increases frequency of bowel movements and softens the stool.
Manufactured by:
Strides Pharma Science Limited
Bengaluru – 5562106, India.
Distributed by
Strides Pharma Inc.
East Brunswick, NJ 08816
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Packets
NDC 59556-762-01
Polyethylene Glycol 3350
Powder for Solution
Osmotic Laxative
- Relieves Occasional Constipation (Irregularity)
- Softens Stool
TAMPER-EVIDENT: DO NOT USE IF FOIL IS OPEN OR BROKEN
- Unflavored
- Sugar Free
- Dissolves in Any Beverage
10 PACKETS
NET WT 0.5 OZ (17g) EACH

10s carton
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Packets
NDC 59556-762-02
Polyethylene Glycol 3350
Powder for Solution
Osmotic Laxative
- Relieves Occasional Constipation (Irregularity)
- Softens Stool
TAMPER-EVIDENT: DO NOT USE IF FOIL IS OPEN OR BROKEN
- Unflavored
- Sugar Free
- Dissolves in Any Beverage
24 PACKETS
NET WT 0.5 OZ (17g) EACH

24s carton
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Original
Prescription
Strength
Polyethylene Glycol 3350
Powder for Solution
Osmotic Laxative
- Relieves Occasional Constipation (Irregularity)
- Softens Stool
TAMPER-EVIDENT: DO NOT USE IF FOIL IS OPEN OR BROKEN
- Unflavored
- Sugar Free
- Dissolves in Any Beverage
NET WT 0.5 OZ (17 g)
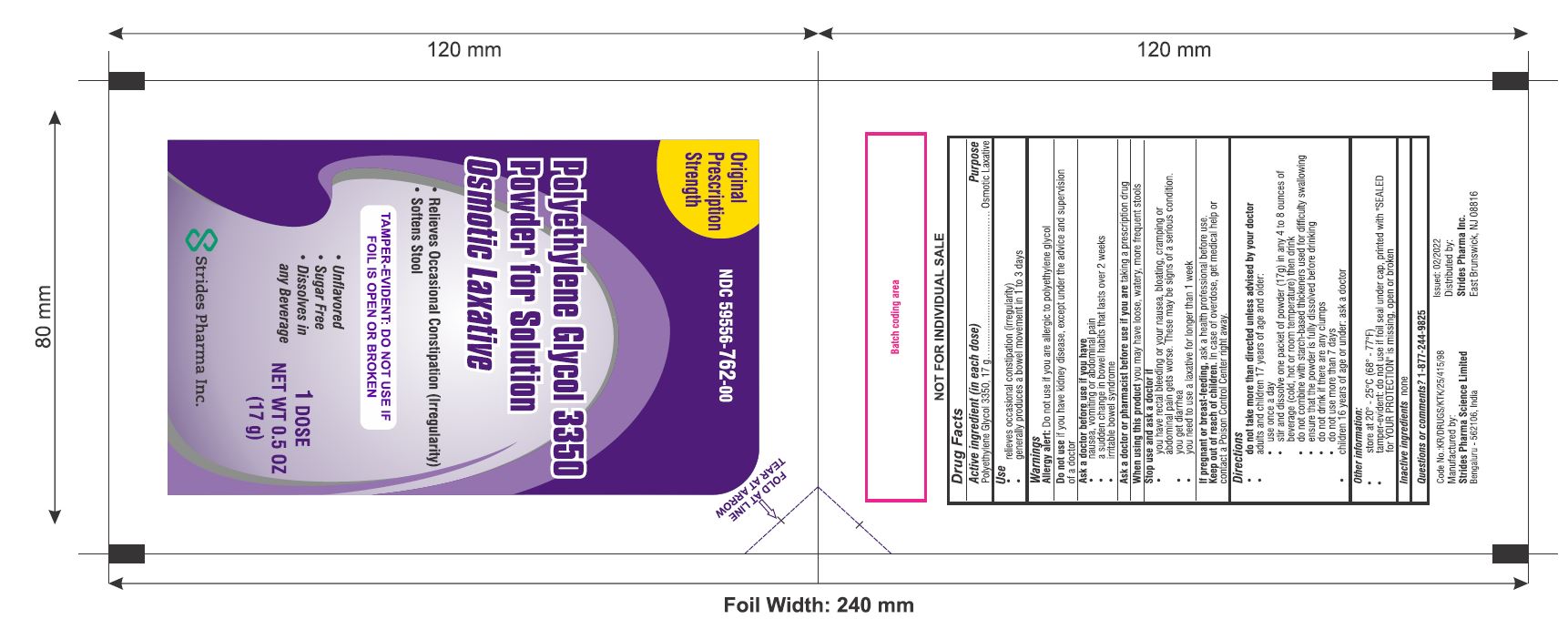
packet
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Original
Prescription
Strength
Polyethylene Glycol 3350
Powder for Solution
Osmotic Laxative
- Relieves Occasional Constipation (Irregularity)
- Softens Stool
TAMPER-EVIDENT: DO NOT USE IF PRINTED FOIL SEAL UNDER CAP IS MISSING, OPEN OR BROKEN
- Unflavored
- Sugar Free
- Dissolves in Any Beverage
ONCE-DAILY
DOSES
NET WT 4.15 OZ (119 g)

119 g CAN - FRONT
119 g BACK [CAN]
119g BACK [CAN]
119g BACK [CAN]
119g BACK [CAN]
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Original
Prescription
Strength
Polyethylene Glycol 3350
Powder for Solution
Osmotic Laxative
- Relieves Occasional Constipation (Irregularity)
- Softens Stool
TAMPER-EVIDENT: DO NOT USE IF PRINTED FOIL SEAL UNDER CAP IS MISSING, OPEN OR BROKEN
- Unflavored
- Sugar Free
- Dissolves in Any Beverage
ONCE-DAILY
DOSES
NET WT 8.3 OZ (238 g)

238 G CAN - FRONT
238 G CAN - BACK
238 G CAN - BACK
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Original
Prescription
Strength
Polyethylene Glycol 3350
Powder for Solution
Osmotic Laxative
- Relieves Occasional Constipation (Irregularity)
- Softens Stool
TAMPER-EVIDENT: DO NOT USE IF PRINTED FOIL SEAL UNDER CAP IS MISSING, OPEN OR BROKEN
- Unflavored
- Sugar Free
- Dissolves in Any Beverage
ONCE-DAILY
DOSES
NET WT 17.9 OZ (510 g)

510 g CAN - FRONT
510 g CAN - BACK