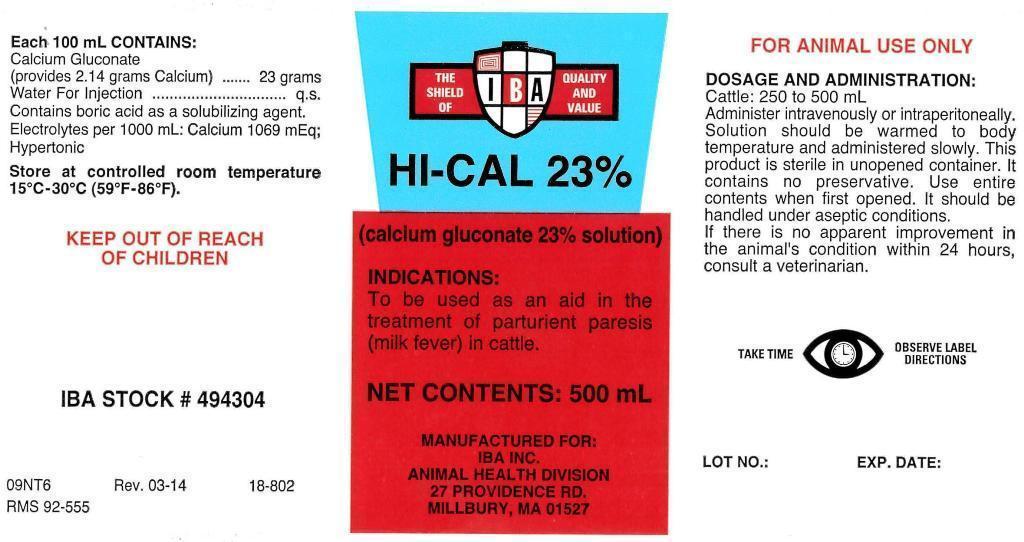
Each 100 mL CONTAINS:
Calcium Gluconate
(provides 2.14 grams Calcium) ...... 23 grams
Water For Injection................................q.s.
IBA STOCK # 494304
09NT6 Rev. 03-14 18-802
RMS 92-555
(calcium gluconate 23% solution)
NET CONTENTS: 500 mL
MANUFACTURED FOR:
IBA INC.
ANIMAL HEALTH DIVISION
27 PROVIDENCE RD.
MILLBURY, MA 01527
LOT NO.: EXP. DATE:
DOSAGE AND ADMINISTRATION:
Cattle: 250 to 500 mL
Administer intravenously or intraperitoneally. Solution should be warmed to body temperature and administered slowly. This product is sterile in unopened container. It contains no preservative. Use entire contents when first opened. it should be handled under aseptic conditions. If there is no apparent improvement in the animal's condition within 24 hours, consult a veterinarian.