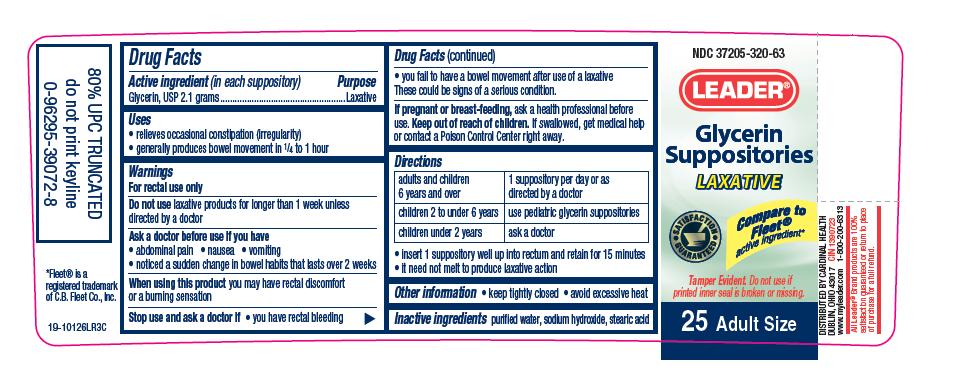
Uses
-
relieves occasional constipation (irregularity)
-
generally produces bowel movement in 1 ⁄ 4 to 1 hour
Ask a doctor before use if you have
-
abdominal pain
-
nausea
-
vomiting
-
noticed a sudden change in bowel habits that lasts over 2 weeks
Stop use and ask a doctor if
-
you have rectal bleeding
-
you fail to have a bowel movement after use of a laxative
These could be signs of a serious condition.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
|
adults and children |
1 suppository per day or as |
|
children 2 to under 6 years |
use pediatric glycerin suppositories |
|
children under 2 years |
ask a doctor |
-
insert 1 suppository well up into rectum and retain for 15 minutes
-
it need not melt to produce laxative action
PRINCIPAL DISPLAY PANEL
NDC 37205-320-63
LEADER®
Glycerin Suppositories
LAXATIVE
Compare to Fleet® active ingredient*
SATISFACTION GUARANTEED
Tamper Evident. Do not use if printed inner seal is broken or missing.
25 Adult Size
DISTRIBUTED BY CARDINAL HEALTH
DUBLIN, OHIO 43017
CIN 1390723
www.myleader.com
1-800-200-6313
All Leader® Brand products are 100% satisfaction guaranteed or return to place of purchase for a full refund.
*Fleet® is a registered trademark of C.B. Fleet Co., Inc.
19-10126LR3C