ZO SKIN HEALTH SULFUR MASQUE- sulfur cream
ZO Skin Health, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
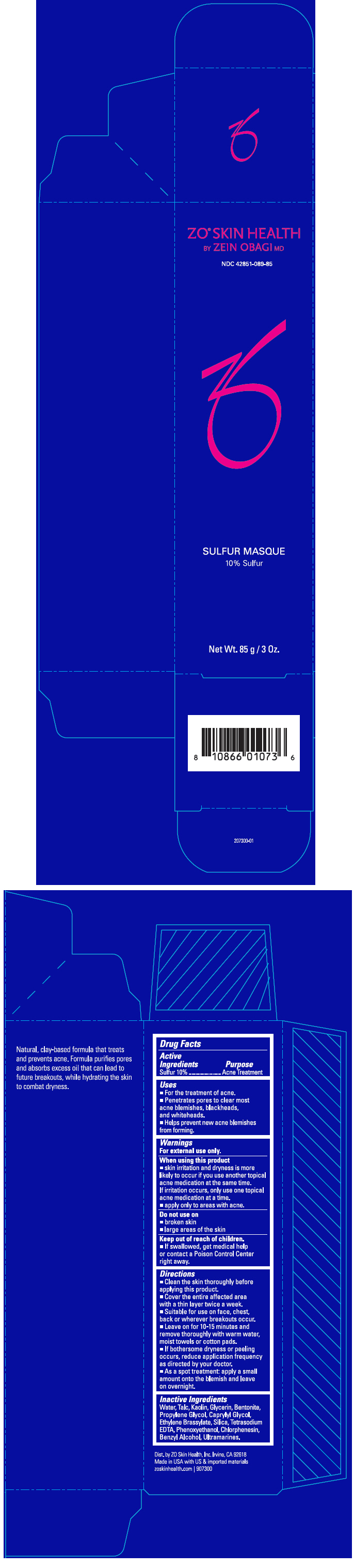
Active Ingredients
Sulfur 10%
Uses
- For the treatment of acne.
- Penetrates pores to clear most acne blemishes, blackheads, and whiteheads.
- Helps prevent new acne blemishes from forming.
Warnings
For external use only.
When using this product
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time.
If irritation occurs, only use one topical acne medication at a time.
- apply only to areas with acne.
Do not use on
- broken skin
- large areas of the skin
Keep out of reach of children.
- If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Clean the skin thoroughly before applying this product.
- Cover the entire affected area with a thin layer twice a week.
- Suitable for use on face, chest, back or wherever breakouts occur.
- Leave on for 10-15 minutes and remove thoroughly with warm water, moist towels or cotton pads.
- If bothersome dryness or peeling occurs, reduce application frequency as directed by your doctor.
- As a spot treatment: apply a small amount onto the blemish and leave on overnight.
Inactive Ingredients
Water, Talc, Kaolin, Glycerin, Bentonite, Propylene Glycol, Caprylyl Glycol, Ethylene Brassylate, Silica, Tetrasodium EDTA, Phenoxyethanol, Chlorphenesin, Benzyl Alcohol, Ultramarines.
Dist. by ZO Skin Health, Inc. Irvine, CA 92618
PRINCIPAL DISPLAY PANEL - 85 g Tube Carton
ZO
® SKIN HEALTH
BY ZEIN OBAGI MD
NDC 42851-089-85
SULFUR MASQUE
10% Sulfur
Net Wt. 85 g / 3 Oz.
ZO Skin Health, Inc.