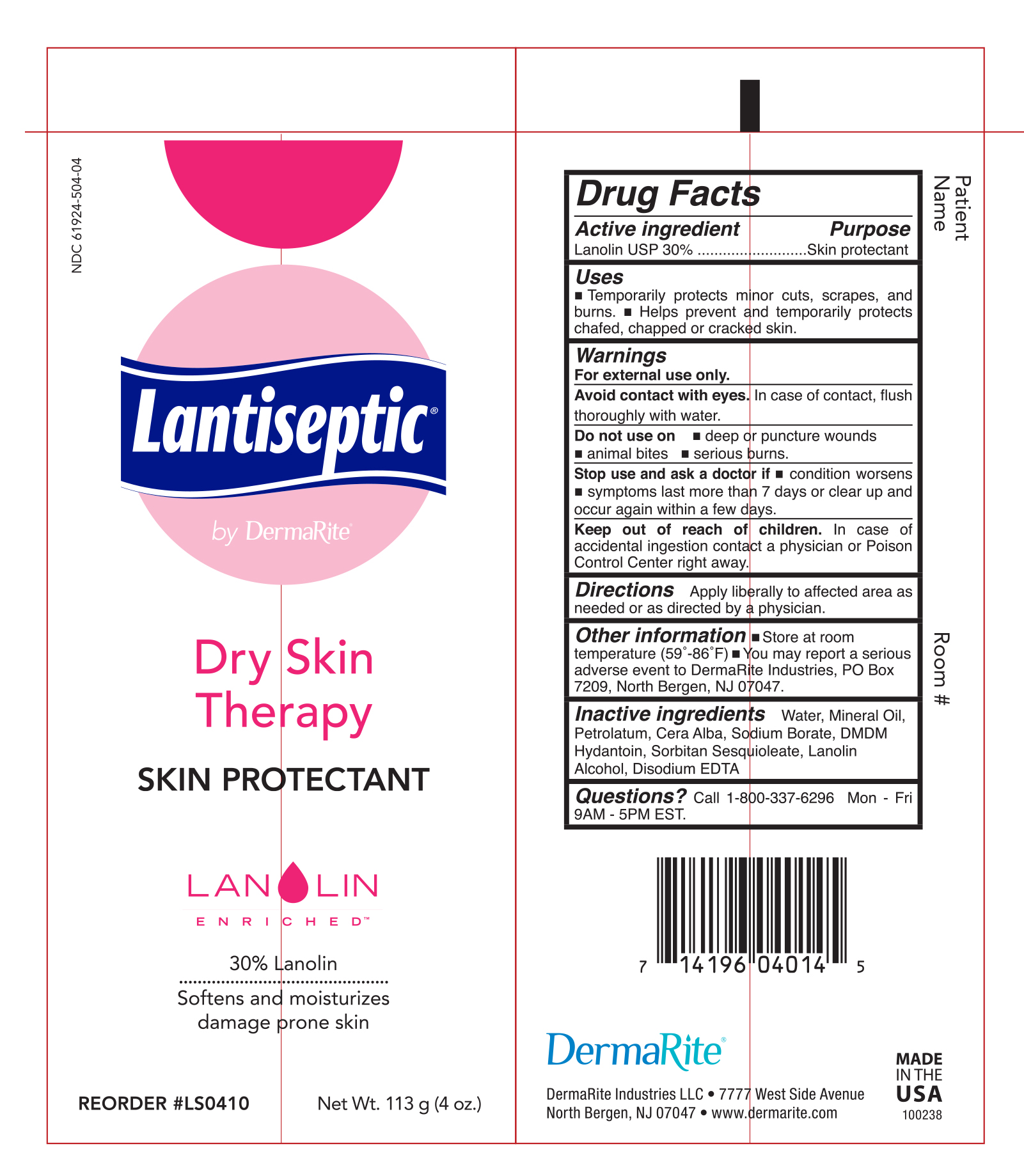
Uses
Temporarily protects minor cuts, scrapes, and burns.
Helps prevent and temporarily protects chafed, chapped or cracked skin.
Warnings
• For external use only.
• Avoid contact with eyes. In case of contact, flush thoroughly with water
- Do not use on
- Deep or puncture wounds
- animal bites
- serious burns
Stop use and ask a doctor if
- condition worsens
- symptoms last more than 7 days or clear up and occur again within a few days
Keep our of reach of children. In case of accidental ingestion contact a physician or Poision Control Center right away.
Other Information
Store at room temperature (59-86°F)
You may report a serious adverse event to DermaRite Industries, PO Box 7209, North Bergen, NJ 07047.