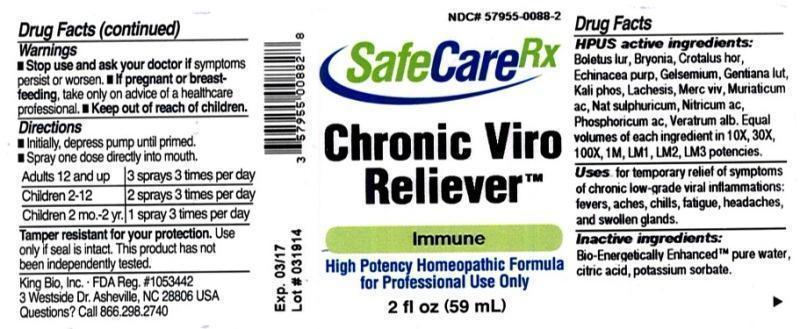
HPUS active ingredients: Boletus luridus, Bryonia, Crotalus horridus, Echinacea purpurea, Gelsemium sempervirens, Gentiana lutea, Kali phosphoricum, Lachesis mutus, Mercurius vivus, Muriaticum acidum, Natrum sulphuricum, Nitricum acidum, Phosphoricum acidum, Veratrum album . Equal volumes of each ingredient in 10X, 30X, 100X, 1M, LM1, LM2, LM3 potencies.
Uses for temporary relief of symptoms of chronic low-grade viral inflammations: fevers, aches, chills, fatigue, headaches, and swollen glands.
Inactive Ingredients
Bio-Energetically Enhanced™ pure water base, Citric acid and Potassium sorbate.
Warning
- Stop use and ask your doctor if symptoms persist or worsen.
- If pregnant or breast-feeding, take only on advice of a healthcare professional.
Directions
- Initially, depress pump until primed.
- Spray one dose directly into mouth.
- Adults 12 and up: 3 sprays 3 times per day.
- Children 2-12: 2 sprays 3 times per day.
- Children 2 mo.-2 yr: 1 spray 3 times per day
Other Information
- Tamper resistant for your protection.
- Use only if seal is intact.
- This product has not been independently tested.