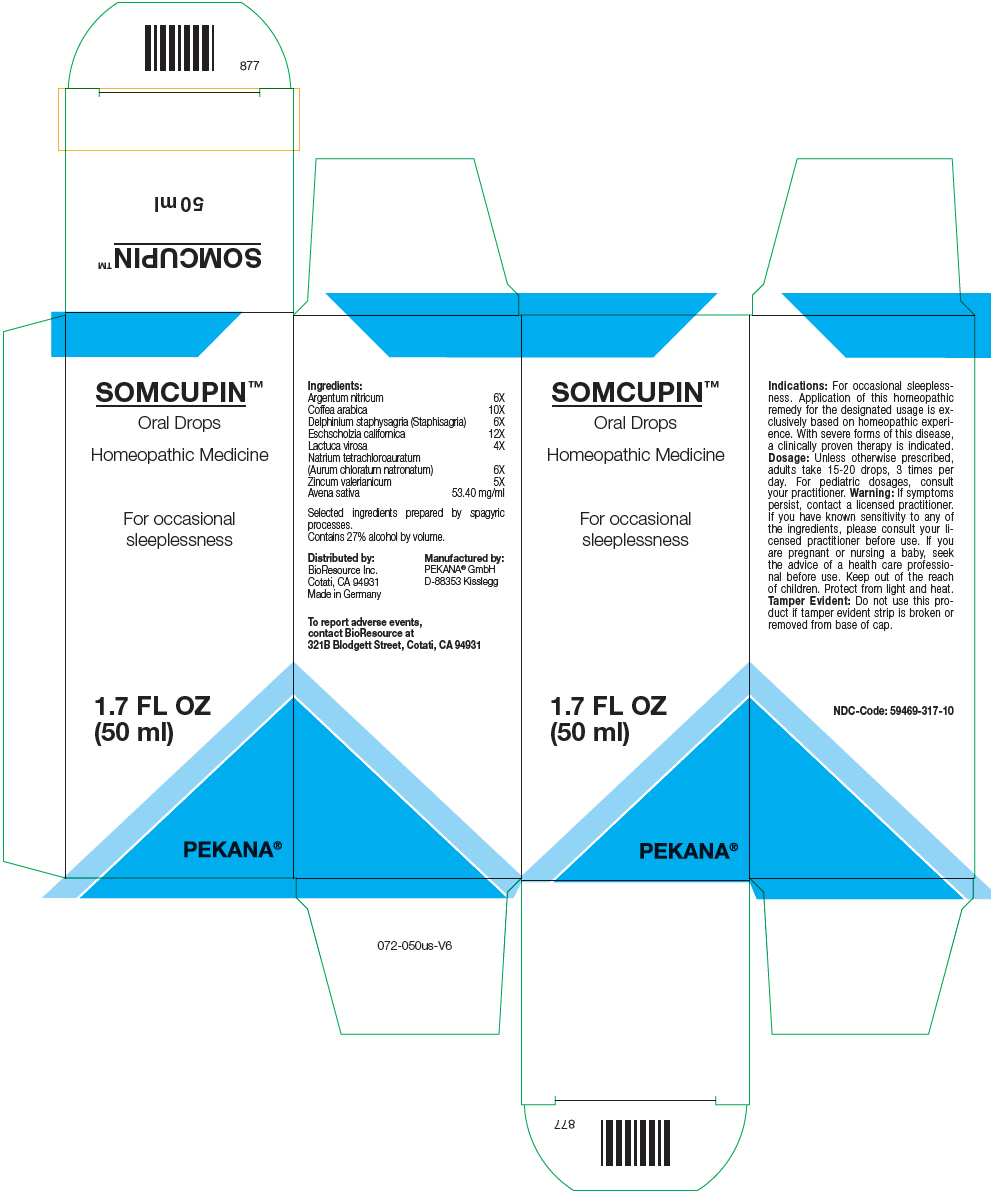
SOMCUPIN- silver nitrate, sodium tetrachloroaurate, arabica coffee bean, delphinium staphisagria seed, lactuca virosa, zinc valerate dihydrate, eschscholzia californica flowering top, and avena sativa flowering top solution/ drops
PEKANA Naturheilmittel GmbH
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
| Ingredients: |
| Argentum nitricum | 6X |
| Coffea arabica | 10X |
| Delphinium staphysagria (Staphisagria) | 6X |
| Eschscholzia californica | 12X |
| Lactuca virosa | 4X |
Natrium tetrachloroauratum
(Aurum chloratum natronatum) | 6X |
| Zincum valerianicum | 5X |
| Avena sativa | 53.40 mg/ml |
Selected ingredients prepared by spagyric processes.
Contains 27% alcohol by volume.
Indications
For occasional sleeplessness. Application of this homeopathic remedy for the designated usage is exclusively based on homeopathic experience. With severe forms of this disease, a clinically proven therapy is indicated.
Dosage
Unless otherwise prescribed, adults take 15-20 drops, 3 times per day. For pediatric dosages, consult your practitioner.
Warning
If symptoms persist, contact a licensed practitioner. If you have known sensitivity to any of the ingredients, please consult your licensed practitioner before use. If you are pregnant or nursing a baby, seek the advice of a health care professional before use.
Keep out of the reach of children.
Protect from light and heat.
Tamper Evident
Do not use this product if tamper evident strip is broken or removed from base of cap.
To report adverse events, contact BioResource at 321B Blodgett Street, Cotati, CA 94931
Distributed by:
BioResource Inc.
Cotati, CA 94931
Manufactured by:
PEKANA® GmbH
D-88353 Kisslegg
PRINCIPAL DISPLAY PANEL - 50 ml Bottle Box
SOMCUPIN™
Oral Drops
Homeopathic Medicine
For occasional
sleeplessness
1.7 FL OZ
(50 ml)
PEKANA®