HEADACHE- hippomane mancinella fruiting leafy twig pellet
Natural Health Supply
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Indications
Indications: To be used for acute self-limiting conditions according to standard homeopathic indications
Active Ingredient
MANCINELLA
Directions
Take at onset of symptoms. Repeat every 2 hours or as needed until relieved. If condition persists or worsens discontinue use and consult a practitioner.
Adults: dissolve 5-10 pellets in 1 oz. of filtered water or take dry by mouth. Children and infants: 1-5 pellets.
Keep Out of Reach of Children
Keep these and all medications out of the reach of children.
Warning
If pregnant or nursing, consult a practitioner before using.
Inactive Ingredients
Inactive Ingredients - Lactose, Sucrose
Purpose
Purpose: Headache
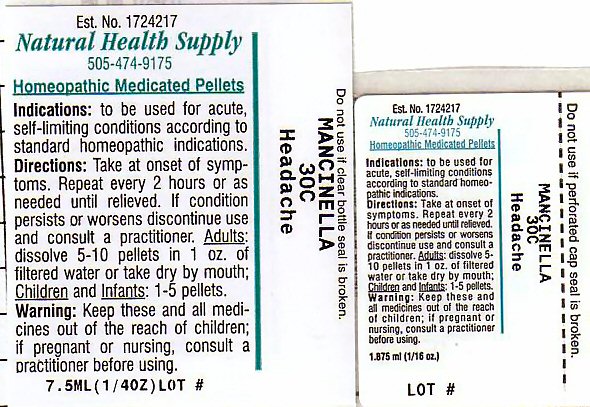
Package Label
Est. No. 1724217
Natural Health Supply
505-474-9175
Homeopathic Medicated Pellets
Do not use if clear bottle seal is broken
MANCINELLA 30C
Heahache
Lot #__________________
res