FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
CAMCEVI is indicated for the treatment of adult patients with advanced prostate cancer.
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
CAMCEVI must be administered by a healthcare provider.
The recommended dose of CAMCEVI is 42 mg administered subcutaneously once every 6 months.
2.2 Preparation and Administration
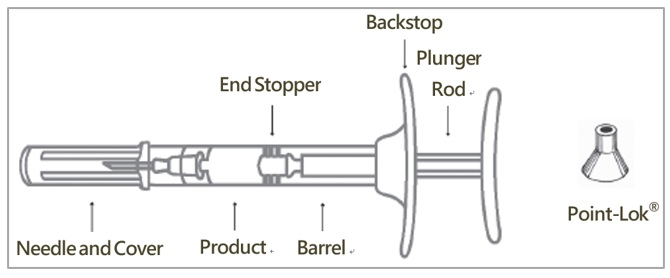
Syringe Assembly
- Remove CAMCEVI kit from refrigerator. Open carton and remove blister.
- Allow prefilled syringe to sit at room temperature for 30 minutes prior to subcutaneous injection.
- Examine all contents of the package. Do not use if any component is damaged.
- Check the expiration date on the syringe. Do not use if the expiration date has passed.
- The use of gloves is recommended during syringe assembly and administration.
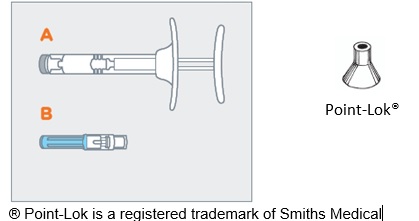
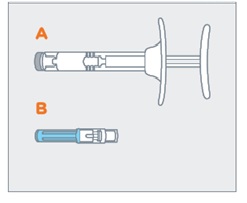
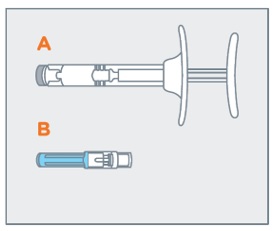
- On a clean, dry surface, remove pre-filled syringe (A) and needle cartridge (B) from the blister carton. Visually inspect the contents prior to use.
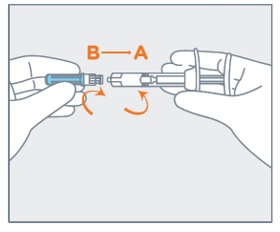
- Remove the gray cap from the syringe (A).
- Twist the clear cap off the bottom of the needle cartridge (B).
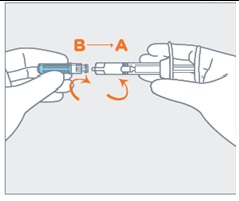
- Attach the needle (B) to the end of the syringe (A) by pushing and turning the needle until firmly connected to the syringe. Do not over twist the needle and strip the threading.
| Remove contents | Syringe Assembly | Assembled Pre-Filled Syringe |
 |  |  |
Administration Procedure
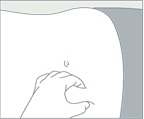
- Choose an injection site on the upper- or mid-abdominal area with sufficient soft or loose subcutaneous tissue that has not recently been used. Clean the injection site with an alcohol swab. Do NOT inject in areas with brawny or fibrous subcutaneous tissue or locations that can be rubbed or compressed (i.e., with a belt or clothing waistband). In addition, avoid applying heat directly to the site of CAMCEVI injection.
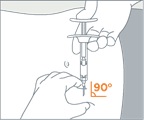
- Pull the blue cover off the needle (B). Use standard sharps safety techniques to avoid needle sticks.
- Use standard aseptic technique when performing the injection. Grab and bunch the skin around the injection site with one hand. Insert the needle at a 90° angle to the skin surface, and then release the bunched skin.
- Inject the full contents of the syringe with a slow and steady push on the plunger, and then withdraw the needle at the same 90° angle used for insertion.
| Prepare the Injection Site | Administer Treatment |
 |  |
Needle Protection
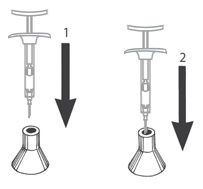
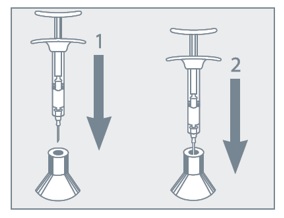
- Do not remove the needle from the syringe. Use the enclosed Point-Lok® device to prevent needle sticks.
- Retrieve the Point-Lok® needle protection device from the Camcevi kit and place it on a secured, flat surface with its largest surface base touching the surface as shown in the diagram below.
- Immediately after use of the needle, gently insert the exposed needle into the Point-Lok® device opening at the top of the Point-Lok® device. (see Figure 1 below)
- Push the needle into the top opening until it is fully inserted into the Point-Lok® device. This action will seal the needle tip and lock the needle firmly into the Point-Lok® device. (see Figure 2 below)
5. After use, place the used syringe with needle protected in a suitable sharps container. Dispose of contaminated product in a safe manner according to Centers for Disease Control and Prevention, USA and Federal/State/Local regulations (EPA,OSHA) and health care facility guidelines or local equivalent.
3 DOSAGE FORMS AND STRENGTHS
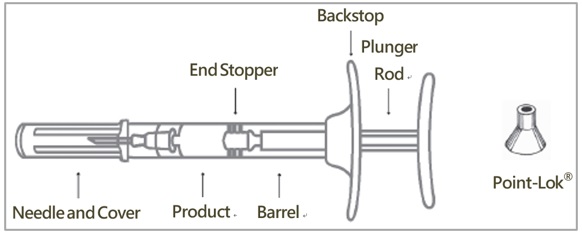
Injectable emulsion: 42 mg leuprolide (equivalent to approximately 48 mg leuprolide mesylate) as a sterile, off-white to pale yellow, viscous, and opalescent emulsion in a single-dose, pre-filled syringe for subcutaneous injection.
4 CONTRAINDICATIONS
CAMCEVI is contraindicated in patients known to be hypersensitive to GnRH, GnRH agonist analogs, or any of the excipients in CAMCEVI. Anaphylactic reactions to GnRH agonist analogs have been reported in the medical literature.
5 WARNINGS AND PRECAUTIONS
5.1 Tumor Flare
CAMCEVI, like other GnRH agonists, causes a transient increase in serum levels of testosterone during the first week of treatment, declining thereafter to baseline levels or below by the end of the second week of treatment. Transient worsening of symptoms, or the occurrence of additional signs and symptoms of prostate cancer, may develop during the first few weeks of CAMCEVI treatment. Patients treated with CAMCEVI may experience a temporary increase in bone pain, which can be managed symptomatically.
As with other GnRH agonists, cases of ureteral obstruction and spinal cord compression have been observed, which may contribute to paralysis with or without fatal complications.
Patients with metastatic vertebral lesions and/or with urinary tract obstruction should be closely observed during the first few weeks of therapy.
5.2 Hyperglycemia and Diabetes
Hyperglycemia and an increased risk of developing diabetes have been reported in men receiving GnRH agonists. Hyperglycemia may represent the development of diabetes mellitus or worsening of glycemic control in patients with diabetes. Monitor blood glucose and/or glycosylated hemoglobin (HbA1c) periodically in patients receiving a GnRH agonist and manage with current practice for treatment of hyperglycemia or diabetes.
5.3 Cardiovascular Diseases
Increased risk of developing myocardial infarction, sudden cardiac death, and stroke has been reported in association with use of GnRH agonists in men. The risk appears low based on the reported odds ratios, and should be evaluated carefully along with cardiovascular risk factors when determining a treatment for patients with prostate cancer. Patients receiving a GnRH agonist should be monitored for symptoms and signs suggestive of development of cardiovascular disease and be managed according to current clinical practice.
5.4 QT/QTc Prolongation
Androgen deprivation therapy may prolong the QT/QTc interval. Providers should consider whether the benefits of androgen deprivation therapy outweigh the potential risks in patients with congenital long QT syndrome, congestive heart failure, frequent electrolyte abnormalities, and in patients taking drugs known to prolong the QT interval. Electrolyte abnormalities should be corrected. Consider periodic monitoring of electrocardiograms and electrolytes.
5.5 Convulsions
Convulsions have been reported in patients receiving GnRH agonists, like CAMCEVI [see Adverse Reactions (6.2)]. Manage patients receiving a GnRH agonist who experience convulsions according to current clinical practice.
5.6 Laboratory Tests
Monitor serum levels of testosterone following injection of CAMCEVI. In the majority of patients treated with CAMCEVI, testosterone levels increased above baseline during the first week, and then declined thereafter to castration levels (<50 ng/dL) within 4 weeks [see Clinical Studies (14) and Adverse Reactions (6)].
5.7 Embryo-Fetal Toxicity
Based on findings in animal studies and mechanism of action, CAMCEVI can cause fetal harm when administered to a pregnant woman. In animal developmental and reproductive toxicology studies, administration of a monthly formulation of leuprolide on day 6 of pregnancy (sustained exposure was expected throughout the period of organogenesis) caused adverse embryo-fetal toxicity in animals at doses less than the human dose based on body surface area using an estimated daily dose. Advise pregnant patients and females of reproductive potential of the potential risk to the fetus [see Use in Specific Population (8.1), Clinical Pharmacology (12.1)].
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
• Tumor Flare [seeWarnings and Precautions (5.1)]
• Hyperglycemia and Diabetes [seeWarnings and Precautions (5.2)]
• Cardiovascular Diseases [seeWarnings and Precautions (5.3)]
• QT/QTc Prolongation [seeWarnings and Precautions (5.4)]
• Convulsions [seeWarnings and Precautions (5.5)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. In an open-label, non-comparative clinical trial (FP01C-13-001), patients with advanced prostate cancer received CAMCEVI administered subcutaneously at a dose of 42 mg on Day 0 and Day 168. Of 137 patients enrolled, 93% received both doses of CAMCEVI.
Serious adverse reactions occurred in 15% of patients who received CAMCEVI, including 1% of patients who experienced subdural hematoma. Fatal adverse reactions occurred in 2% of patients, including cerebrovascular accident (0.7%) and pulmonary embolism (0.7%).
The most common adverse reactions (>10%) occurring during a median follow-up duration of 336 days were hot flush, hypertension, injection site reactions, upper respiratory tract infections, musculoskeletal pain, fatigue, and pain in extremity.
Table 1 summarizes the adverse reactions in FP01C-13-001.
|
||
| Adverse Reaction | N = 137 | |
| All Grades
(%) | Grade 3-4
(%) |
|
| Vascular disorders | ||
| Hot flush* | 50 | 0 |
| Hypertension† | 15 | 0 |
| General disorders and administration site conditions | ||
| Injection site reactions‡ | 11 | 0 |
| Fatigue§ | 10 | 0 |
| Infections and infestations | ||
| Upper respiratory tract infection¶ | 11 | 0 |
| Musculoskeletal and connective tissue disorders | ||
| Musculoskeletal pain# | 11 | 0 |
| Pain in extremity | 10 | 0 |
| Arthralgia | 7 | 0 |
| Renal and urinary disorders | ||
| Micturition urgencyÞ | 6 | 0 |
| Nocturia | 6 | 0 |
| Nervous system disorders | ||
| Dizzinessß | 5 | 0.7 |
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of leuprolide. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
During postmarketing surveillance, which includes other dosage forms and other patient populations, the following adverse reactions were reported.
Allergic Conditions: anaphylactoid or asthmatic process, rash, urticaria, and photosensitivity reactions
Cardiovascular System: hypotension, myocardial infarction, pulmonary embolism
Central/Peripheral Nervous System: convulsion, peripheral neuropathy, spinal fracture/paralysis
Endocrine System: pituitary apoplexy, diabetes
Hepato-biliary disorder: drug-induced liver injury
Hematologic: white blood cells
Psychiatric: mood swings, including depression, suicidal ideation and attempt
Respiratory, thoracic and mediastinal disorder: interstitial lung disease
Musculoskeletal System: decreased bone density, tenosynovitis-like symptoms, fibromyalgia
Skin and Subcutaneous: injection site reactions
Urogenital System: prostate pain
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings in animal studies and mechanism of action, CAMCEVI can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available data in pregnant women to inform the drug-associated risk. In animal developmental and reproductive toxicology studies, administration of a monthly formulation of leuprolide on day 6 of pregnancy (sustained exposure was expected throughout the period of organogenesis) caused adverse embryo-fetal toxicity in animals at doses less than the human dose based on body surface area using an estimated daily dose (see data). Advise pregnant patients and females of reproductive potential of the potential risk to the fetus.
Data
Animal Data
Major fetal malformations were observed in developmental and reproductive toxicology studies in rabbits after a single administration of a monthly formulation of leuprolide administered on day 6 of pregnancy at test dosages of 0.00024, 0.0024, and 0.024 mg/kg (approximately 1/1500 to 1/15 the human dose based on body surface area using an estimated daily dose in animals and humans). Since a depot formulation was utilized in the study, a sustained exposure to leuprolide was expected throughout the period of organogenesis and to the end of gestation. Similar studies in rats did not demonstrate an increase in fetal malformations, however, there was increased fetal mortality and decreased fetal weights with the two higher doses of the monthly formulation of leuprolide in rabbits and with the highest dose in rats.
8.2 Lactation
Risk Summary
The safety and efficacy of CAMCEVI have not been established in females. There is no information regarding the presence of leuprolide in human milk, the effects on the breastfed child, or the effects on milk production. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in a breastfed child, a decision should be made to discontinue breastfeeding or discontinue the drug, taking into account the importance of the drug to the mother.
8.3 Females and Males of Reproductive Potential
Infertility
Males
Based on findings in animals and mechanism of action, CAMCEVI may impair fertility in males of reproductive potential [see Clinical Pharmacology (12.1), Nonclinical Toxicology (13.1)].
11 DESCRIPTION
CAMCEVI is a sterile formulation of leuprolide mesylate for subcutaneous injection. CAMCEVI is designed to deliver approximately 42 mg of leuprolide over 6 months.
Leuprolide mesylate is a synthetic nonapeptide analog of naturally occurring GnRH and is a GnRH agonist. The analog possesses greater potency than the natural hormone. The chemical name is 5-oxo-L-prolyl-L-histidyl-L-tryptophyl-L-seryl-L-tyrosyl-D-leucyl-L-leucyl-L-arginyl-N-ethyl-L-prolinamide mesylate (salt) with the following structural formula. The pH of 50 mg/mL solution of leuprolide mesylate in water is approximately 5.7.
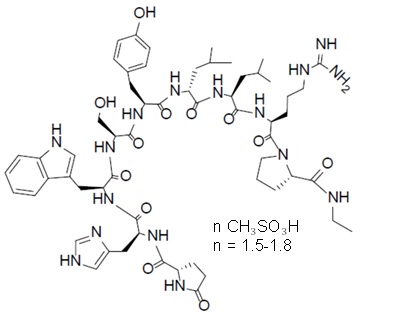
CAMCEVI is supplied as a kit with a pre-filled, single-dose, sterile syringe for subcutaneous injection. Each pre-filled syringe delivers 42 mg leuprolide (equivalent to approximately 48 mg leuprolide mesylate), poly(D, L-lactide) (184 mg) polymer and N-methyl-2-pyrrolidone (136 mg).
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Leuprolide, a GnRH agonist, acts as an inhibitor of gonadotropin secretion. Animal and human studies indicate that following an initial stimulation of gonadotropins, chronic administration of leuprolide results in suppression of ovarian and testicular steroidogenesis. This effect is reversible upon discontinuation of drug therapy.
In humans, subcutaneous administration of single daily doses of leuprolide result in an initial increase in circulating levels of LH and FSH, leading to a transient increase in levels of the gonadal steroids (testosterone and dihydrotestosterone in males). However, continuous daily administration of leuprolide results in decreased levels of LH and FSH. In males, testosterone is reduced to below castration levels. These decreases generally occur within 2 to 4 weeks after initiation of treatment, and castration levels of testosterone in prostatic cancer patients have been demonstrated for periods of up to 5 years.
12.2 Pharmacodynamics
Mean serum testosterone concentrations transiently increased, then fell to below castrate threshold levels (≤ 50 ng/dL) within 3 weeks following administration of the dose of leuprolide, and generally remained below castrate thresholds levels throughout treatment.
12.3 Pharmacokinetics
Leuprolide concentration is variable, exhibiting an initial rapid increase followed by a rapid decline over the first 3 days before reaching steady concentrations for the duration of the dosing interval. The mean serum leuprolide Cmax was 94.5 and 99 ng/mL following the first and second doses of CAMCEVI, respectively. The mean serum concentration was maintained at 0.497–2.57 and 0.507–2.39 ng/ml after Day 3 post the first and second doses, respectively. The mean AUC0-6 mon was 224 and 268 day.ng/mL following the first and second doses of CAMCEVI, respectively.
Absorption
The median Tmax of leuprolide was 3.2 and 2.1 hours following the first and second doses of CAMCEVI, respectively.
Distribution
The mean steady‑state volume of distribution of leuprolide was 27 L following an intravenous bolus in healthy male volunteers. Protein binding of leuprolide ranged from 43% to 49% in vitro.
Elimination
The mean systemic clearance was 7.6 L/h and terminal elimination half‑life of approximately 3 hours following an intravenous bolus of leuprolide in healthy male volunteers.
Metabolism
Administration of radiolabeled leuprolide was metabolized to smaller inactive peptides which may then be further catabolized.
Specific Populations
No clinically significant differences in the systemic exposure of leuprolide were observed based on age (51 to 88 years), race/ethnicity (White, Black, Asian), or body weight (54 to 134 kg). The effect of renal or hepatic impairment on the pharmacokinetics of leuprolide has not been evaluated.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Two‑year carcinogenicity studies were conducted with leuprolide in rats and mice. In rats, a dose-related increase of benign pituitary hyperplasia and benign pituitary adenomas was noted at 24 months when the drug was administered subcutaneously at high daily doses (0.6 to 4 mg/kg). There was a significant but not dose‑related increase of pancreatic islet‑cell adenomas in females and of testicular interstitial cell adenomas in males (highest incidence in the low dose group). In mice, no pituitary abnormalities were observed at a dose as high as 60 mg/kg for 2 years. Patients have been treated with leuprolide for up to 3 years with doses as high as 10 mg/day and for 2 years with doses as high as 20 mg/day without demonstrable pituitary abnormalities.
Mutagenicity studies have been performed with leuprolide using bacterial and mammalian systems. These studies provided no evidence of mutagenic potential.
Leuprolide may reduce male and female fertility. Administration of leuprolide to male and female rats at doses of 0.024, 0.24, and 2.4 mg/kg as monthly depot formulation for up to 3 months (approximately as low as 1/30 of the human dose based on body surface area using an estimated daily dose in animals and humans) caused atrophy of the reproductive organs, and suppression of reproductive function. These changes were reversible upon cessation of treatment.
14 CLINICAL STUDIES
The efficacy of CAMCEVI was evaluated in an open label, single arm, multinational study FP01C-13-001 (NCT02234115) in patients with advanced prostate carcinoma who have a baseline morning serum testosterone level >150 ng/dL and Eastern Cooperative Oncology Group performance status ≤ 2. CAMCEVI was administered subcutaneously at a dose of 42 mg initially on Day 0 and on Week 24.
The population (n = 137) had a median age of 71 years (range 51 to 88) and was 90% White, 6% Black, and 4% Asian. Disease stage was distributed as follows: 23% metastatic (M1), 27% locally advanced (T3/4 NX M0 or any T N1 M0), 26% localized (T1 or T2 N0 M0), and 24% not classifiable. The median testosterone concentration at baseline was 440 ng/dL.
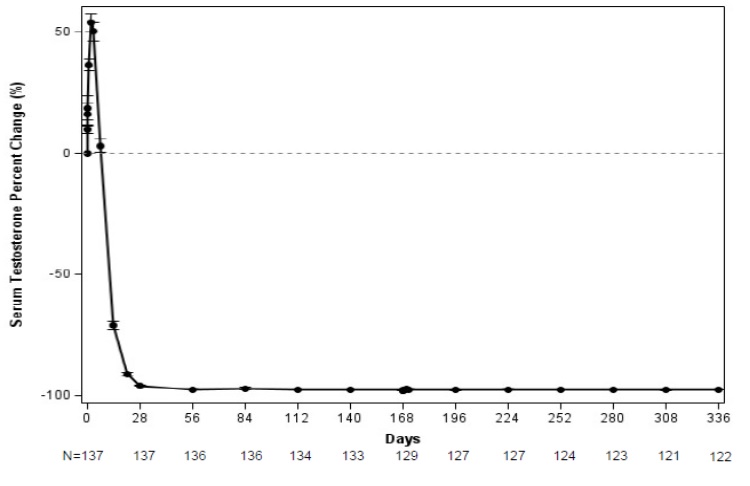
The major efficacy outcome measure was medical castration rate, defined as achieving and maintaining serum testosterone suppression to ≤ 50 ng/dL by Week 4 through Week 48 of treatment. Following the first injection of CAMCEVI, serum testosterone levels were suppressed to ≤ 50 ng/dL by Week 4 (+/-7 days) in 98.5% of the patients; and from Week 4 through Week 48 in 97.0% of patients (95% CI: 92.2-98.9) estimated using the Kaplan-Meier method. The time course of percent change from baseline in testosterone suppression are shown in Figure 1. The percentage of patients with testosterone suppression to ≤ 20 ng/dL was 69.3% on Day 28.
Figure 1 CAMCEVI Mean (95% CI) Percentage Change from Baseline in Serum Testosterone Concentration Over Time (N =137)
In the clinical trial, PSA levels were monitored and were lowered on average by 51% after 4 weeks after administration of CAMCEVI, 83% after 3 months and remained suppressed throughout the 48 weeks of treatment. These PSA results should be interpreted with caution because of the heterogeneity of the patient population studied. No evidence has shown that the rapidity of PSA decline correlates with clinical benefit.
16 HOW SUPPLIED/STORAGE AND HANDLING
CAMCEVI is a sterile, off-white to pale yellow, viscous and opalescent injectable emulsion supplied in a kit as a single-dose, pre-filled syringe. CAMCEVI is available as follows:
| Kit Contents | NDC |
| Injectable emulsion in a pre-filled syringe containing 42 mg leuprolide for subcutaneous injection, a sterile 18 gauge needle, a Point-Lok® needle protection device, and Instructions for Use. | 72851-042-01 |
Store CAMCEVI at 2°C–8°C (36°F–46°F). Protect CAMCEVI from light by storing in the original package until time of use. Do not freeze or shake.
The rubber used in syringe tip cap and plunger stopper is not made of natural rubber latex.
17 PATIENT COUNSELING INFORMATION
Hypersensitivity
- Inform patients that if they have experienced hypersensitivity with other GnRH agonist drugs like CAMCEVI, CAMCEVI is contraindicated [see Contraindications (4)].
Tumor Flare
- Inform patients that CAMCEVI can cause tumor flare during the first weeks of treatment. Inform patients that the increase in testosterone can cause an increase in urinary symptoms or pain. Advise patients to contact their healthcare provider if uretral obstruction, spinal cord compression, paralysis, or new or worsened symptoms occur after beginning CAMCEVI treatment [see Warnings and Precautions (5.1)].
Hyperglycemia and Diabetes
- Advise patients that there is an increased risk of hyperglycemia and diabetes with CAMCEVI therapy. Inform patients that periodic monitoring for hyperglycemia and diabetes is required when being treated with CAMCEVI [see Warnings and Precautions (5.2)].
Cardiovascular Disease
- Inform patients that there is an increased risk of myocardial infarction, sudden cardiac death, and stroke with CAMCEVI treatment. Advise patients to immediately report signs and symptoms associated with these events to their healthcare provider for evaluation [see Warnings and Precautions (5.3)].
QT/QTc Prolongation
- Inform patients that CAMCEVI can cause QT/QTc prolongation. Advise patients to immediately contact their healthcare provider in the event of syncope, presyncopal symptoms, or cardiac palpitations [see Warnings and Precautions (5.4)].
- ·
Convulsions
- Inform patients that there is an increased risk of convulsions with CAMCEVI treatment. Advise patients to immediately contact their healthcare provider if they experience convulsions [see Warnings and Precautions (5.5)].
Injection Site Reactions
- Inform patients that injection site related adverse reactions may occur such as transient burning/stinging, pain, bruising, and redness. Advise patients to contact their healthcare provider if they experience rash or severe injection site reactions [see Adverse Reactions (6.1)].
Urogenital Disorders
- Advise patients that CAMCEVI may cause impotence.
Infertility
- Inform patients that CAMCEVI may cause infertility [seeUse In Specific Populations (8.3)].
Manufactured for:
Foresee Pharmaceuticals Co., Ltd.
9F-2, No. 19-3, Sanchong Rd., Nangang Dist., Taipei City 115, Taiwan
By:
Fareva Pau 1
Avenue du Bearn, 64320 Idron, France
Instructions for Use
 | You must read these complete instructions before you administer CAMCEVI for the first time.
CAMCEVI must be administered by a healthcare provider. |
| What is CAMCEVI? |
CAMCEVI is a subcutaneous injection that provides long-acting gonadotropin releasing hormone (GnRH) agonist to patients. CAMCEVI contains a biodegradable polymer formulation dissolved in a biocompatible solvent that provides sustained release of leuprolide. CAMCEVI is supplied as a kit containing one sterile, pre-filled syringe for subcutaneous injection, one sterile 18-gauge needle, one Point-Lok® needle protection device, and Instructions for Use. |
|
| Dosage | The recommended dosing is 1 subcutaneous injection (42 mg leuprolide) every 6 months. | |
| Before you begin to prepare CAMCEVI Injection |
Read these critical instructions. Do not substitute any of the components from the kit for administration. CAMCEVI is packaged in a blister in the kit. Check to make sure the kit contains:
|
|
| Bring CAMCEVI to room temperature | STEP | To bring CAMCEVI to room temperature: |
| 1 |
Remove CAMCEVI kit from refrigerator. Open carton and remove blister. Allow prefilled syringe to sit at room temperature for 30 minutes prior to subcutaneous injection. |
|
| Prepare the syringe | STEP | Follow these steps to prepare CAMCEVI for use: |
| 1 | Examine all contents of the package. Do not use if any component is damaged. | |
| 2 | Check the expiration date on the syringe. Do not use if the expiration date has passed. | |
| 3 | The use of gloves is recommended during syringe assembly and administration. | |
| 4 |
On a clean, dry surface, remove pre-filled syringe (A) and needle cartridge (B) from the blister carton. Visually inspect the contents prior to use.
|
|
| 5 | Remove the gray cap from the syringe (A). Twist the clear cap off the bottom of the needle cartridge (B). | |
| 6 |
Attach the needle (B) to the end of the syringe (A) by pushing and turning the needle until firmly connected to the syringe. Do not over twist the needle and strip the threading.
Assembled Pre-Filled Syringe
|
|
| Select an injection site | STEP | To select an injection site, use these guidelines: |
| 1 |
Choose an injection site on the upper- or mid-abdominal area with sufficient soft or loose subcutaneous tissue that has not recently been used. Clean the injection site with an alcohol swab.
Do NOT inject in areas with brawny or fibrous subcutaneous tissue or locations that can be rubbed or compressed (i.e., with a belt or clothing waistband). In addition, avoid applying heat directly to the site of Camcevi injection. |
|
| Inject CAMCEVI | STEP | Follow these steps to inject CAMCEVI: |
| 1 | Pull the blue cover off the needle (B). Use standard sharps safety techniques to avoid needle sticks. | |
| 2 |
Use standard aseptic technique when performing the injection. Grab and bunch the skin around the injection site with one hand. Insert the needle at a 90° angle to the skin surface, and then release the bunched skin.  |
|
| 3 | Inject the full contents of the syringe with a slow and steady push on the plunger, and then withdraw the needle at the same 90° angle used for insertion. | |
| 4 |
Do not remove the needle from the syringe. Use the enclosed Point-Lok® device to prevent needle sticks. Retrieve the Point-Lok® needle protection device from the Camcevi kit and place it on a secured, flat surface with its largest surface base touching the surface as shown in the diagram below. Immediately after use of the needle, gently insert the exposed needle into the Point-Lok® device opening at the top of the Point-Lok® device. (see Figure 1 below) Push the needle into the top opening until it is fully inserted into the Point-Lok® device. This action will seal the needle tip and lock the needle firmly into the Point-Lok® device. (see Figure 2 below)
After use, place the used syringe with needle protected in a suitable sharps container. Dispose of contaminated product in a safe manner according to Centers for Disease Control and Prevention, USA and Federal/State/Local regulations (EPA, OSHA) and health care facility guidelines or local equivalent. |
|
Manufactured for:
Foresee Pharmaceuticals Co. Ltd.
9F-2, No. 19-3, Sanchong Rd., Nangang Dist., Taipei City 115, Taiwan
By:
Fareva Pau 1
Avenue du Bearn, 64320 Idron, France
PRINCIPAL DISPLAY PANEL- Carton
NDC 72851-042-01
Rx Only
CAMCEVITM
(leuprolide) injectable emulsion
42 mg single-dose prefilled syringe
Front

Back
PRINCIPAL DISPLAY PANEL- Syringe Label
NDC 72851-042-01
Rx Only
CAMCEVITM
(leuprolide) injectable emulsion
42 mg single-dose prefilled syringe
For Subcutaneous Injection Only

PRINCIPAL DISPLAY PANEL- Outer Label
NDC 72851-042-01
Rx Only
CAMCEVITM
(leuprolide) injectable emulsion
42 mg single-dose prefilled syringe
This bilster carton contans:
- One sterile 42 mg pre-filled syringe
- One sterile 18-gauge needle
Single Dose Administration Kit with prefilled syringe
For Subcutaneous Injection Only once Every 6 Months