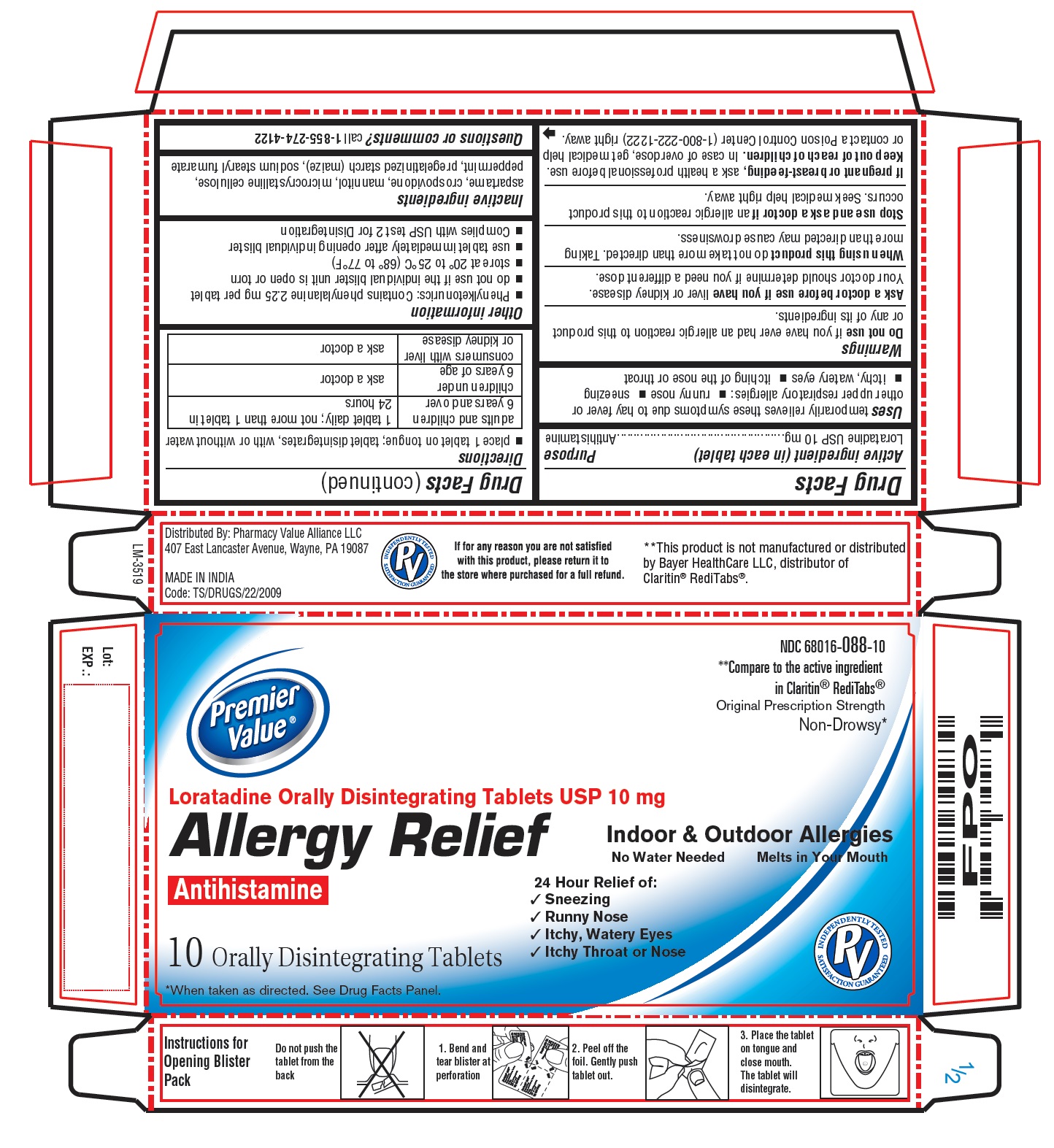
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
Ask a doctor before use if you have
liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
do not take more than directed. Taking more than directed may cause drowsiness.
Stop use and ask a doctor if
an allergic reaction to this product occurs. Seek medical help right away.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
- place 1 tablet on tongue; tablet disintegrates, with or without water
| adults and children 6 years and over | 1 tablet daily; not more than 1 tablet in 24 hours |
| children under 6 years of age | ask a doctor |
| consumers with liver or kidney disease | ask a doctor |
Other information
- Phenylketonurics: Contains phenylalanine 2.25 mg per tablet
- do not use if the individual blister unit is open or torn
- store at 20° to 25°C (68° to 77°F)
- use tablet immediately after opening individual blister
- Complies with USP test 2 for Disintegration
Inactive ingredients
aspartame, crospovidone, mannitol, microcrystalline cellulose, peppermint, pregelatinized starch (maize), sodium stearyl fumarate
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 10 mg, Blister Carton 10 Orally Disintegrating Tablets
NDC 68016-088-10
**Compare to the active ingredient
in Claritin® RediTabs®
Original Prescription Strength
Non-Drowsy*
Premier Value ®
Loratadine Orally Disintegrating Tablets USP 10 mg
Allergy Relief
Antihistamine
Indoor & Outdoor Allergies
No Water Needed Melts in Your Mouth
24 Hour Relief of:
- Sneezing
- Runny Nose
- Itchy, Watery Eyes
- Itchy Throat or Nose
10 Orally Disintegrating Tablets
*When taken as directed. See Drug Facts Panel.