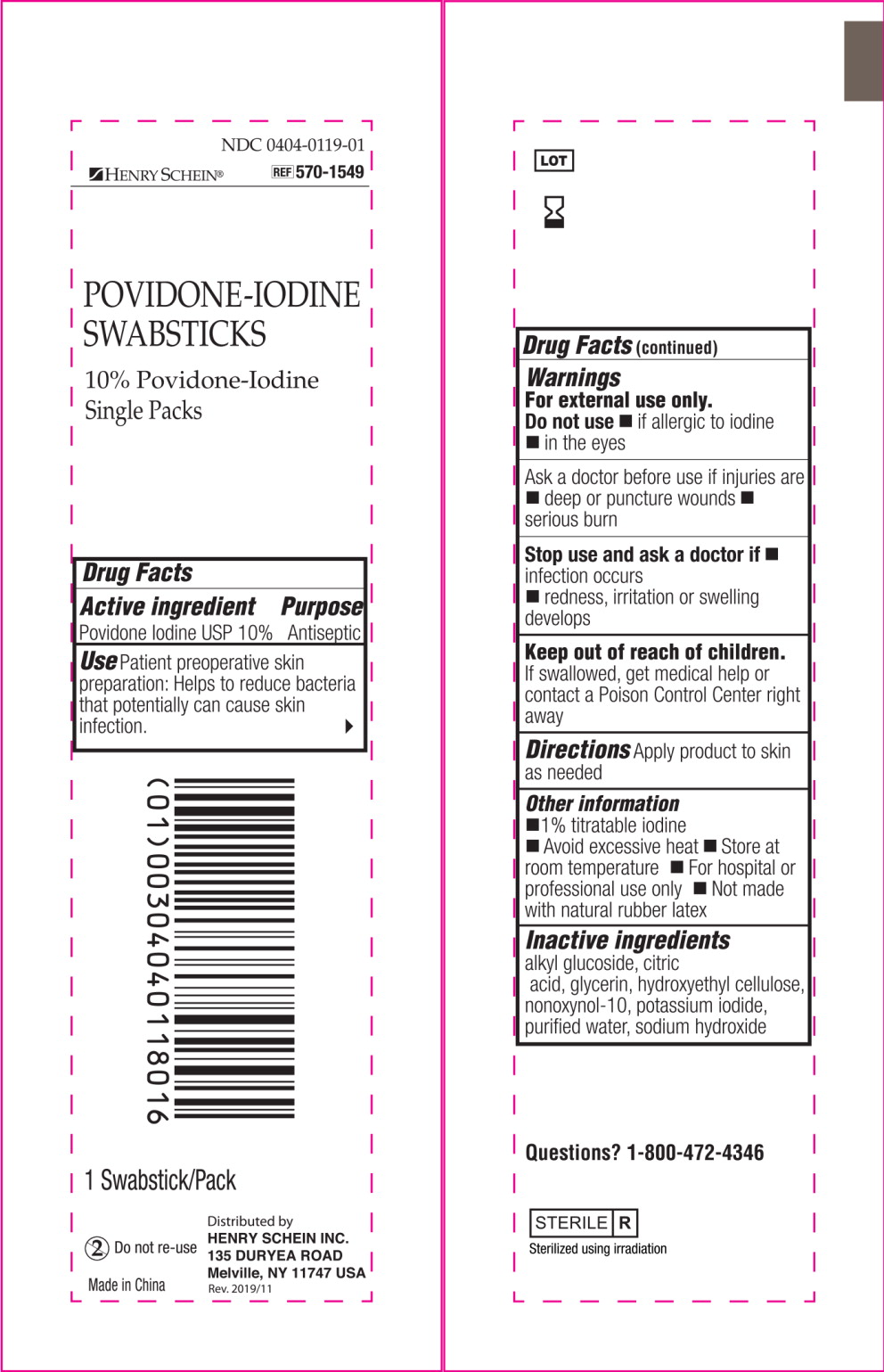
Active ingredient
Povidone Iodine USP 10%
Use
Patient preoperative skin preparation: Helps to reduce bacteria that potentially can cause skin infection.
Warnings
For external use only.
Do not use
- if allergic to iodine
- in the eyes
Ask a doctor before use if injuries are
- deep or puncture wounds
- serious burn
Stop use and ask a doctor if
- infection occurs
- redness, irritation or swelling develops
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away
Directions
Apply product to skin as needed
Other information
- 1 % titratable iodine
- Avoid excessive heat
- Store at room temperature
- For hospital or professional use only
- Not made with natural rubber latex
Inactive ingredients
alkyl glucoside, citric acid, glycerin, hydroxyethyl cellulose, nonoxynol-10, potassium iodide, purified water, sodium hydroxide
Questions?
1-800-472-4346
Principal Display Panel - 2.3 mL Item Label
NDC 0404-0119-01
HENRY SCHEIN
®
REF 570-1549
POVIDONE-IODINE
SWABSTICKS
10% Povidone-Iodine
Single Packs
1 Swabstick/Pack
Do not re-use
Henry Schein, Inc.