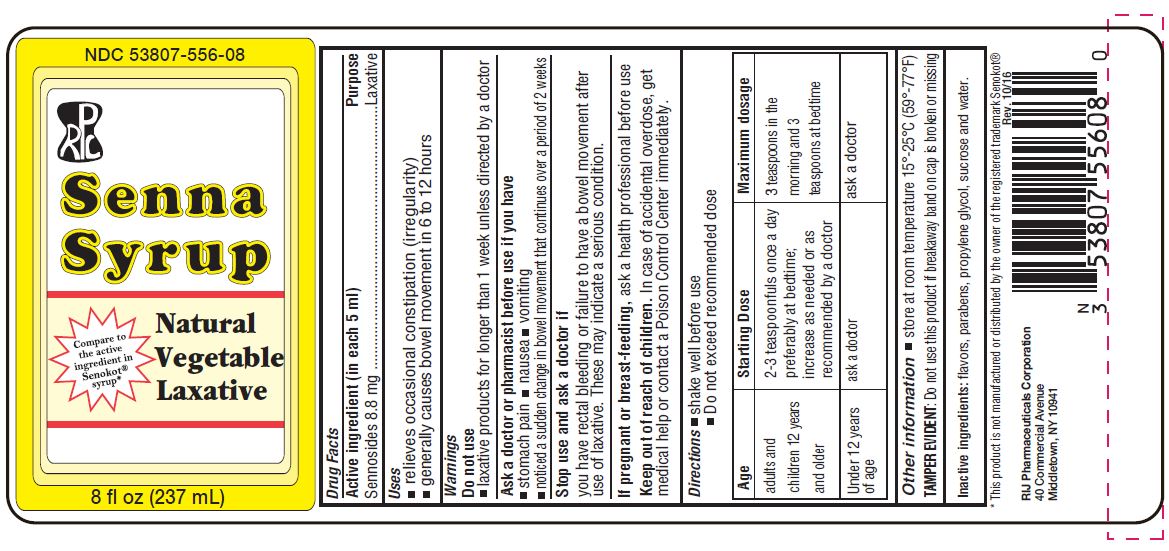
SENNA- sennosides a and b syrup
Rij Pharmaceutical Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient (in each teaspoonful)
Sennosides 8.8 mg
Uses
- relieves occasional constipation (irregularity)
- generally produces bowel movement in 6-12 hours
Warnings
Do not use
- laxative products for longer than 1 week unless directed by a doctor
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel movements that continues over a period of 2 weeks
Stop use and ask a doctor if you have rectal bleeding or fail to have bowel movement after use of laxative. These may indicate a serious condition.
If pregnant or breast-feeding, ask a professional before use.
Keep out of reach of children. In case of accidental overdose, get medical help or contact a Poison Control Center right away.
Directions
- shake well before use
- Do not exceed recommended dose
| Age | Starting Dose | Maximum dosage |
| adults and children 12 years and older | 2 - 3 teaspoons once a day preferable at bedtime; increase as needed or as recommended by a doctor | 3 teaspoons in the morning and 3 teaspoons at bedtime |
| Under 12 years of age | ask a doctor | ask a doctor |
Other information
- store at room temperature 15°- 25°C (59°- 77°F)
TAMPER EVIDENT: Do not use this product if breakaway band on cap is broken or missing.
Inactive ingredients
flavors, parabens, propylene glycol, sucrose, and water.
RIJ Pharmaceuticals Corporation
40 Commercial Avenue
Middletown, NY 10941
PRINCIPAL DISPLAY PANEL - 237 mL Bottle Label
NDC 53807-556-08
Senna Syrup
Senna Syrup
Natural Vegetable Laxative
8 fl. oz. (237 mL)