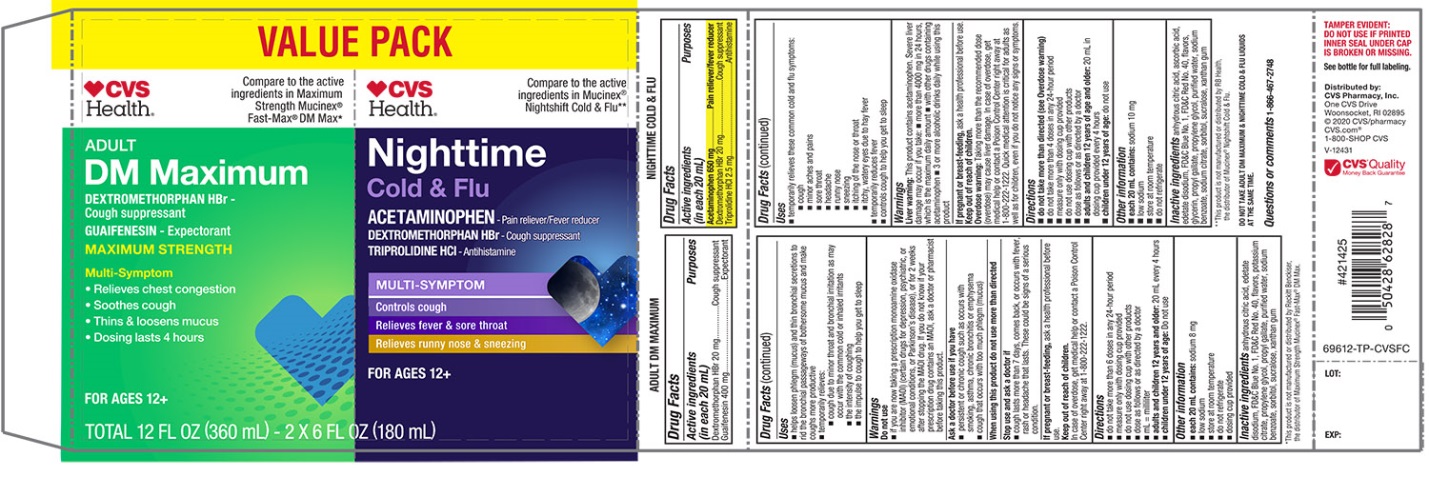
| Active ingredients (in each 20 mL)
Maximum Strength Mucus Relief DM Max | Purposes |
|---|---|
|
Dextromethorphan HBr 20 mg |
Cough suppressant |
|
Guaifenesin 400 mg |
Expectorant |
| Active ingredients (in each 20 mL) | Purposes |
|---|---|
| Nighttime Cold & Flu | |
|
Acetaminophen 650 mg |
Pain reliever/fever reducer |
|
Dextromethorphan HBr 20 mg |
Cough suppressant |
|
Triprolidine HCl 2.5 mg |
Antihistamine |
Uses
MAXIMUM STRENGTH MUCUS RELIEF DM
- •
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
- •
- temporarily relieves
- •
- cough due to minor throat and bronchial irritation as may occur with the common cold or inhaled irritants
- •
- the intensity of coughing
- •
- the impulse to cough to help you get to sleep
Warnings
Do not use
- •
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- ▪
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis or emphysema
- ▪
- cough that occurs with too much phlegm (mucus)
Directions
- •
- do not take more than 6 doses in any 24-hour period
- •
- measure only with dosing cup provided
- •
- do not use dosing cup with other products
- •
- dose as follows or as directed by a doctor
- •
- mL = milliliter
- •
- adults and children 12 years and older: 20 mL every 4 hours
- •
- children under 12 years of age: Do not use
Other information
- •
- each 20 mL contains: sodium 8 mg
- •
- low sodium
- •
- store at room temperature
- •
- do not refrigerate
- •
- dosing cup provided
Inactive ingredients (Maximum strength mucus relief DM)
anhydrous citric acid, edetate disodium, FD&C Blue No.1, FD&C Red No. 40, flavors, potassium citrate, propylene glycol, propyl gallate, purified water, sodium benzoate, sorbitol, sucralose, xanthan gum
Uses (Nighttime Cold and Flu)
- ▪
- temporarily relieves these common cold and flu symptoms:
- ▪
- cough
- ▪
- minor aches and pains
- ▪
- sore throat
- ▪
- headache
- ▪
- runny nose
- ▪
- sneezing
- ▪
- itching of the nose or throat
- ▪
- itchy, watery eyes due to hay fever
- ▪
- temporarily reduces fever
- ▪
- controls cough to help you get to sleep
Warnings
Liver warnings: This product contains acetaminophen. Severe liver damage may occur if you take\
- ▪
- more than 4000 mg in 24 hours, which is the maximum daily amount
- ▪
- with other drugs containing acetaminophen
- ▪
- 3 or more alcoholic drinks daily while using this product
If pregnant or breast feeding
Ask a health professional before use
Keep Out of Reach of Children
Overdose warning: Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Centre right away at 1-800-222-1222.
Quick medical attention is critical for adults as well as for children, even if you do not notice any signs
Directions
- ▪
- do not take more than directed (see overdose warnings
- ▪
- do not take more than 4 doses in any 24-hour period
- ▪
- measure only with dosing cup provided
- ▪
- do not use dosing cup with other products
- ▪
- dose as follows or as directed by a doctor
- ▪
- adults and children 12 years of age and older: 20 ml in dosing cup provided every 4 hours
- ▪
- children under 12 years of age: do not use
Inactive ingredients (Overnight Cold & Flu)
anhydrous citric acid, ascorbic acid, edetate disodium, FD&C Blue No. 1, FD&C Red No. 40, flavors, glycerin, propyl gallate, propylene glycol, purified water, sodium benzoate, sodium citrate, sorbitol, sucralose, xanthan gum
PRINCIPAL DISPLAY PANEL - Kit Carton
VALUE PACK
NDC 69842-696-12
Compare to the active ingredients Maximum Strength Mucinex® Fast Max® DM Max*
Mucus Relief DM
Dextromethorphan HBr • Cough Suppressant
Guaifenesin • Expectorant
MAXIMUM STRENGTH
MULTI-SYMPTOM
- •
- Controls Cough
- •
- Relieves chest congestion
- •
- Thins & loosens mucus
- •
- 4 hour dosing
For Ages 12+
*This product is not manufactured or distributed by Reckitt Benckiser, the distributor of Maximum Strength Mucinex® Fast-Max® DM Max.
Compare to Mucinex® Nightshift Cold & Flu Active Ingredients**
Overnight Cold & Flu
ACETAMINOPHEN • PAIN RELIEVER/FEVER REDUCER
DEXTROMETHORPHAN HBR • COUGH SUPPRESSANT
TRIPROLIDINE HCL • ANTIHISTAMINE
Night Time
Relief for Better Morning
Maximum Strength per 4-hour dose
- •
- Cough
- •
- Fever
- •
- Sore Throat
- •
- Runny Nose
- •
- Sneezing
For Ages 12+
2 – 6 FL OZ (180 mL) BOTTLES / TOTAL 12 FL OZ (360 mL)
††These product is not manufactured or distributed by Reckitt Benckister Health, distributor of Maximum Strength Mucinex® Fast Max© DM Max & Mucinex© Nightshift Cold & Flu.
|
DO NOT TAKE ADULT DM MAXIMUM & NIGHTTIME COLD & FLU LIQUIDS AT THE SAME TIME. |
|
TAMPER EVIDENT: DO NOT USE IF PRINTED INNER SEAL UNDER CAP IS BROKEN OR MISSING.; |
See bottle for full labeling
Distributed By:
CVS Pharmacy, Inc.
One CVS Drive
Woonsocket, RI 02895
© 2020 CVS/pharmacy
CVS.com®
1-800-SHOP CVS
V-12431
CVS® Quality
Money Back Guarantee