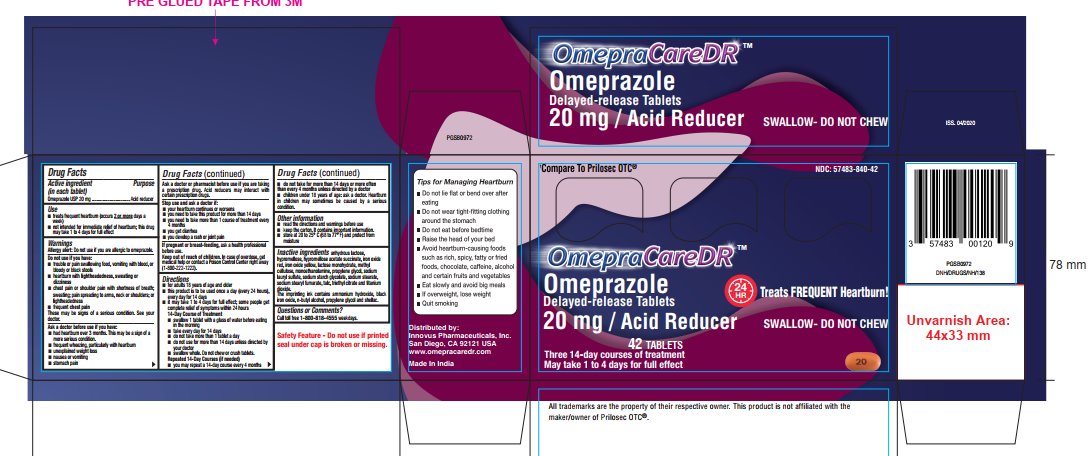
Use
■ treats frequent heartburn (occurs 2 or moredays a week)
■ not intended for immediate relief of heartburn; this drug may take 1 to 4 days for full effect
Directions
- •
- for adults 18 years of age and older
■ this product is to be used once a day (every 24 hours), every day for 14 days
■ it may take 1 to 4 days for full effect; some people get complete relief of symptoms within 24 hours
14-Day Course of Treatment
■ swallow 1 tablet with a glass of water before eating in the morning
■ take every day for 14 days
■ do not take more than 1 tablet a day
■ do not use for more than 14 days unless directed by your doctor
■ swallow whole. Do not chew or crush tablets.
Repeated 14-Day Courses (if needed)
■ you may repeat a 14-day course every 4 months
■ do not take for more than 14 days or more often than every 4 months unless directed by a doctor
■ children under 18 years of age: ask a doctor. Heartburn in children may sometimes be caused by a serious condition
Inactive ingredients
anhydrous lactose, hypromellose, hypromellose acetate succinate, iron oxide red, iron oxide yellow, lactose monohydrate, methyl cellulose, monoethanolamine, propylene glycol, sodium lauryl sulfate, sodium starch glycolate, sodium stearate, sodium stearyl fumarate, talc, triethyl citrate and titanium dioxide. The imprinting ink contains ammonium hydroxide, black iron oxide, n-butyl alcohol, propylene glycol and shellac.