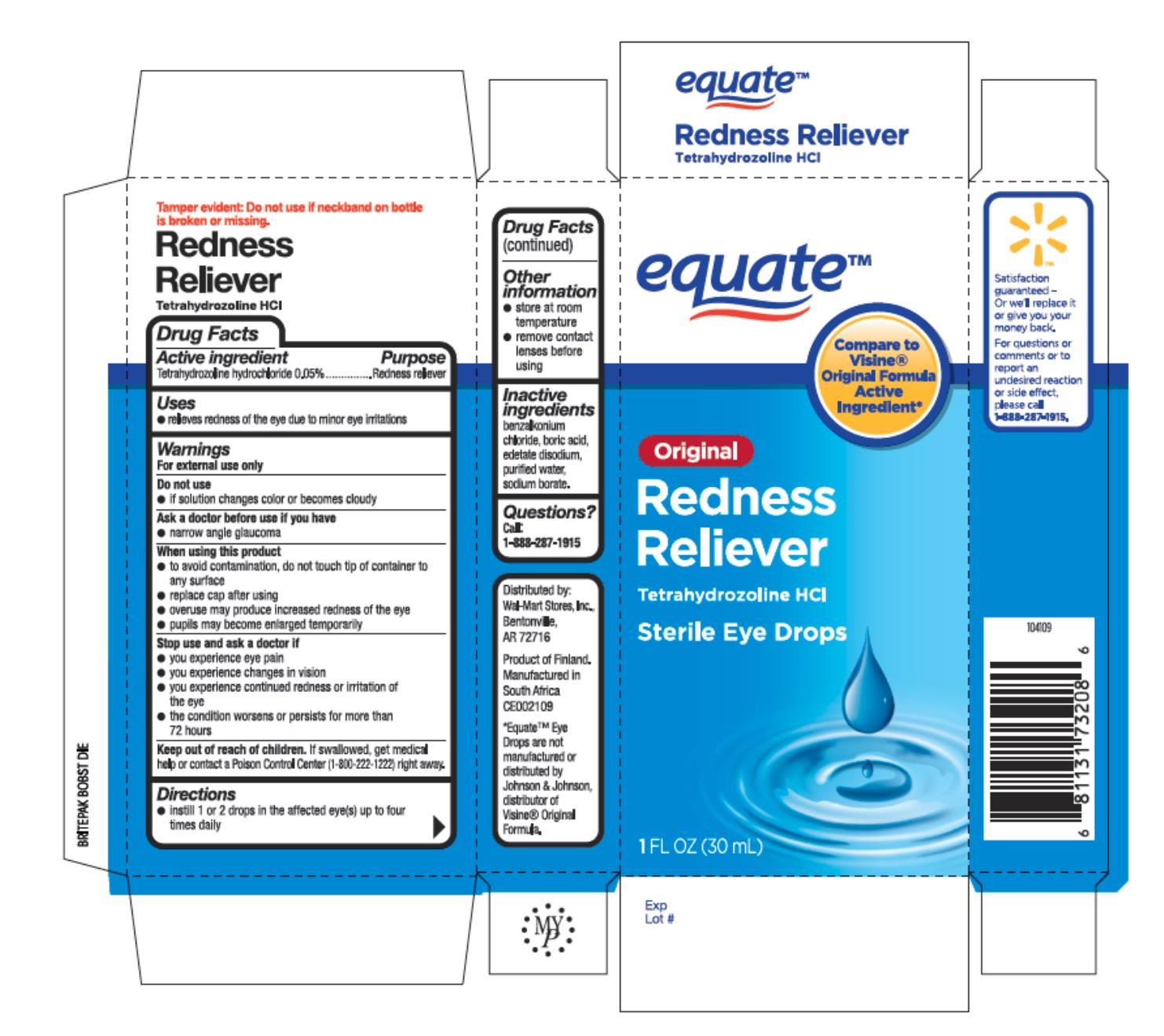
EQUATE EYE DROPS- tetrahyrozoline hydrochloride liquid
Prestige Brands Holdings, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Tetrahydrozoline Hydrochloride 0.05%
Uses
- relieves redness of the eye due to minor eye irritations
Warnings
For external use only
Do not use
- if solution changes color or becomes cloudy
Ask a doctor before use if you have
When using this product
- to avoid contamination, do not touch tip of container to any surface
- replace cap after using
- overuse may produce increased redness of the eye
- pupils may become enlarged temporarily
Stop use and ask a doctor if
- you experience eye pain
- you experience changes in vision
- you experience continued redness or irritation of the eye
- the condition worsens or persists for more than 72 hours
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
- instill 1 to 2 drops in the affected eye(s) up to four times daily
Other information
- store at room temperature
- remove contact lenses before using
Inactive ingredients
benzalkonium chloride, boric acid, edetate disodium, purified water, sodium borate
Questions?
1-888-287-1915
Principal Display Panel
Equate®
Original Redness Reliever
Tetrahydrozoline HCI
Sterile Eye Drops
1 FL OZ (30 mL)
Principal Display Panel
Equate®
Original Redness
Reliever
Tetrahydrozoline HCI
Sterile Eye Drops
0.5 FL OZ (15 mL)

Prestige Brands Holdings, Inc.

