FULL PRESCRIBING INFORMATION
WARNING: EMBOLIC and THROMBOTIC EVENTS
- Thromboembolic events have been reported during postmarketing surveillance following infusion of FEIBA, particularly following the administration of high doses and/or in patients with thrombotic risk factors.
- Monitor patients receiving FEIBA for signs and symptoms of thromboembolic events.
1 INDICATIONS AND USAGE
FEIBA is an Anti-Inhibitor Coagulant Complex indicated for use in hemophilia A and B patients with inhibitors for:
- Control and prevention of bleeding episodes
- Perioperative management
- Routine prophylaxis to prevent or reduce the frequency of bleeding episodes.
FEIBA is not indicated for the treatment of bleeding episodes resulting from coagulation factor deficiencies in the absence of inhibitors to coagulation factor VIII or coagulation factor IX.
2 DOSAGE AND ADMINISTRATION
For intravenous use after reconstitution only.
2.1 Dose
A guide for dosing FEIBA is provided in Table 1.
| Dose (unit/kg) | Frequency of Doses (hours) | Duration of Therapy | |
|---|---|---|---|
| Control and Prevention of Bleeding | |||
| Joint Hemorrhage | 50 – 100 | 12 | Until pain and acute disabilities are improved. |
| Mucous Membrane Bleeding | 50 – 100 | 6 | At least 1 day or until bleeding is resolved. |
| Soft Tissue Hemorrhage (e.g., retroperitoneal bleeding) | 100 | 12 | Until resolution of bleed. |
| Other Severe Hemorrhage (e.g., CNS bleeds) | 100 | 6 – 12 | Until resolution of bleed. |
| Perioperative Management | |||
| Preoperative | 50 – 100 | One time dose | Immediately prior to surgery. |
| Postoperative | 50 – 100 | 6 – 12 | Until resolution of bleed and healing are achieved. |
| Routine Prophylaxis | |||
| 85 | Every other day | ||
- Dosage and duration of treatment depend on the location and extent of bleeding, and the patient's clinical condition. Careful monitoring of replacement therapy is necessary in cases of major surgery or life-threatening bleeding episodes.
- Each vial of FEIBA contains the labeled amount of factor VIII inhibitor bypassing activity in units.
- Base the dose and frequency of FEIBA on the individual clinical response. Clinical response to treatment with FEIBA may vary by patient and may not correlate with the patient's inhibitor titer.
- Record the name of the patient and batch number of the product in order to maintain a link between the patient and the batch of the product.
- Do not exceed a single dose of 100 units per kg body weight or a daily dose of 200 units per kg body weight [see Warnings and Precautions (5.1)].
2.2 Preparation and Reconstitution
- Use aseptic technique throughout the entire reconstitution process.
- If the patient uses more than one vial per injection, reconstitute each vial according to the following instructions.
- Allow the vials of FEIBA and Sterile Water for Injection (diluent) to reach room temperature, if refrigerated.
- Remove the plastic caps from the concentrate and diluent vials.
- Wipe the stoppers of both vials with a sterile alcohol swab and allow them to dry prior to use.
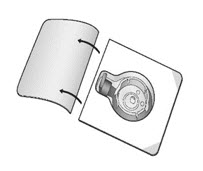
- Open the package of BAXJECT II Hi-Flow device by peeling away the lid completely without touching the inside (Fig. A). Do not remove the device from the package. Do not touch the clear spike.
- Place the diluent vial on a flat and solid surface. Turn the package over and insert the clear plastic spike through the diluent stopper by pressing straight down (Fig. B).
- Grip the BAXJECT II Hi-Flow device package at the edges and pull the package off the device (Fig. C). Do not remove the blue protective cap from the BAXJECT II Hi-Flow device. Do not touch the purple spike.
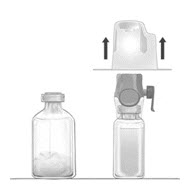
- Turn the system over, so that the diluent vial is on top. Quickly insert the purple spike of the BAXJECT II Hi-Flow device fully into the FEIBA vial. The vacuum will draw the diluent into the FEIBA vial (Fig. D). The connection of the two vials should be done expeditiously to close the open fluid pathway created by the first insertion of the spike to the diluent vial.
- Gently swirl (do not shake) the vial until FEIBA is completely dissolved. Make sure that FEIBA has been dissolved completely; otherwise, active material will not pass through the device filter. The reconstituted solution should be inspected visually for particulate matter before administration. The solution should be discarded if it is not clear or is discolored.
- After reconstitution of FEIBA is complete, the injection or infusion should begin immediately and must be completed within three hours following reconstitution. Do not refrigerate after reconstitution. Discard unused portion.
Figure A
 | Figure B
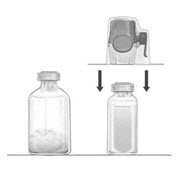 |
Figure C
 | Figure D
 |
2.3 Administration
For intravenous injection or intravenous infusion after reconstitution only.
- Inspect the reconstituted FEIBA solution visually for particulate matter and discoloration prior to administration. The appearance of the solution should be colorless to slightly yellowish. Do not use if particulate matter or discoloration is observed.
- Flush venous access lines with isotonic saline prior to and after infusion of FEIBA. Do not administer in the same tubing or container with other medicinal products.
- Use plastic Luer lock syringes because protein such as FEIBA tends to stick to the surface of all-glass syringes.
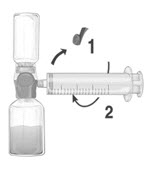
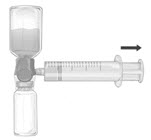
- Remove the blue protective cap from the BAXJECT II Hi-Flow device. Tightly connect the syringe to the BAXJECT II Hi-Flow device (DO NOT DRAW AIR INTO THE SYRINGE) by turning the syringe in clockwise direction until stop position. Use of a Luer lock syringe is highly recommended to ensure a tight connection between the syringe and the BAXJECT II Hi-Flow device (Fig. E).
- Invert the system so that the dissolved FEIBA product is on top. Draw the dissolved product carefully into the syringe by pulling the plunger back slowly to avoid foaming (Fig. F).
- Ensure that the tight connection between the BAXJECT II Hi-Flow device and the syringe is maintained.
- Disconnect the syringe.
- Attach a suitable needle and inject or infuse intravenously at a rate that does not exceed 10 units per kg of body weight per minute. A syringe pump may be used to control the rate of administration. For a patient with a body weight of 75 kg, this corresponds to an infusion rate up to 15 mL per minute.
Figure E
 | Figure F
 |
3 DOSAGE FORMS AND STRENGTHS
FEIBA is available as a lyophilized powder in single-dose glass vials containing nominally 500, 1000, or 2500 units per vial.
4 CONTRAINDICATIONS
- Known anaphylactic or severe hypersensitivity reactions to FEIBA or any of its components, including factors of the kinin generating system.
- Disseminated intravascular coagulation (DIC).
- Acute thrombosis or embolism (including myocardial infarction).
5 WARNINGS AND PRECAUTIONS
5.1 Embolic and Thrombotic Events
Thromboembolic events (including venous thrombosis, pulmonary embolism, myocardial infarction, and stroke) can occur with FEIBA, particularly following the administration of high doses (above 200 units per kg per day) and/or in patients with thrombotic risk factors [see Adverse Reactions (6)].
Patients with DIC, advanced atherosclerotic disease, crush injury, septicemia, or concomitant treatment with recombinant factor VIIa have an increased risk of developing thrombotic events due to circulating tissue factor or predisposing coagulopathy. Potential benefit of treatment with FEIBA should be weighed against the potential risk of these thromboembolic events.
Monitor patients receiving more than 100 units per kg of body weight of FEIBA for the development of DIC, acute coronary ischemia and signs and symptoms of other thromboembolic events. If clinical signs or symptoms occur, such as chest pain or pressure, shortness of breath, altered consciousness, vision, or speech, limb or abdomen swelling and/or pain, discontinue the infusion and initiate appropriate diagnostic and therapeutic measures.
The safety and efficacy of FEIBA for breakthrough bleeding in patients receiving emicizumab has not been established. Cases of thrombotic microangiopathy (TMA) were reported in a clinical trial where subjects received FEIBA as part of a treatment regimen for breakthrough bleeding following treatment with emicizumab.6 Consider the benefits and risks with FEIBA if considered required for patients receiving emicizumab prophylaxis. If treatment with FEIBA is required for patients receiving emicizumab, the hemophilia treating physician should closely monitor for signs and symptoms of TMA. In FEIBA clinical studies thrombotic microangiopathy (TMA) has not been reported.
5.2 Hypersensitivity Reactions
Hypersensitivity and allergic reactions, including severe anaphylactoid reactions, can occur following the infusion of FEIBA. The symptoms include urticaria, angioedema, gastrointestinal manifestations, bronchospasm, and hypotension. These reactions can be severe and systemic (e.g., anaphylaxis with urticaria and angioedema, bronchospasm, and circulatory shock). Other infusion reactions, such as chills, pyrexia, and hypertension have also been reported. If signs and symptoms of severe allergic reactions occur, immediately discontinue administration of FEIBA and provide appropriate supportive care.
5.3 Transmission of Infectious Agents
Because FEIBA is made from human plasma it may carry a risk of transmitting infectious agents, e.g., viruses, and the variant Creutzfeldt-Jakob disease (vCJD) agent, and theoretically, the Creutzfeldt-Jakob disease (CJD) agent. The risk has been minimized by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections and by inactivating and removing certain viruses during the manufacturing process [see Description (11)]. Despite these measures, the product may still potentially transmit human pathogenic agents. There is also the possibility that unknown infectious agents may still be present.
All infections thought by a physician to have been possibly transmitted by this product should be reported by the physician or other healthcare providers to Takeda Pharmaceuticals U.S.A., Inc. at 1-877-TAKEDA-7 (1-877-825-3327) (in the U.S.) and /or to FDA Med Watch (1-800-FDA-1088 or www.fda.gov/medwatch).
5.4 Presence of Isohemagglutinins and Interference with Laboratory Tests
FEIBA contains blood group isohemagglutinins (anti-A and anti-B). Passive transmission of antibodies to erythrocyte antigens, e.g., A, B, D, may interfere with some serological tests for red cell antibodies, such as antiglobulin test (Coombs test).
6 ADVERSE REACTIONS
The most frequently reported adverse reactions observed in >5% of subjects in the prophylaxis trial were anemia, diarrhea, hemarthrosis, hepatitis B surface antibody positive, nausea, and vomiting.
The serious adverse reactions seen with FEIBA are hypersensitivity reactions and thromboembolic events, including stroke, pulmonary embolism and deep vein thrombosis.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety assessment of FEIBA is based on the review of the data from two prospective clinical trials in which FEIBA was used for the treatment of acute bleeding episodes and a prospective trial that compared the use of FEIBA prophylactically versus on-demand treatment.
The adverse reactions reported from two prospective clinical trials in which FEIBA was used for the treatment of acute bleeding episodes were chills, chest pain, chest discomfort, dizziness, dysgeusia, dyspnea, hypoesthesia, increase of inhibitor titer (anamnestic response), nausea, pyrexia, and somnolence. Specifically, the first trial was a multicenter randomized, double-blind trial in 15 hemophilia A subjects with inhibitors to factors VIII. The second trial was a multicenter FEIBA study conducted in 44 hemophilia A subjects with inhibitors, 3 hemophilia B subjects with inhibitors and 2 acquired factor VIII inhibitor subjects. Of the 489 infusions used to treat acute bleeds during the second trial, 18 (3.7%) caused minor transient reactions of chills, fever, nausea, dizziness and dysgeusia. Out of 49 subjects, 10 (20%) had a rise in their inhibitor titers after treatment with FEIBA. Five of these subjects (50%) had increases that were tenfold or more, and 3 (30%) of these subjects received factor VIII or IX concentrates within 2 weeks prior to treatment with FEIBA. These anamnestic rises were not associated with decreased efficacy of FEIBA.
Table 2 lists the adverse reactions in >5% of subjects reported in the randomized, prospective prophylaxis trial comparing FEIBA prophylaxis with on-demand treatment in 36 hemophilia A and B subjects with inhibitors to factors VIII or IX3. The trial population included 33 (92%) subjects with hemophilia A and 3 (8.3%) subjects with hemophilia B. Four (11%) subjects were ≥7 to <12 years of age, 5 (14%) were ≥12 to <16 years of age, and 27 (75%) were ≥16 years of age. A total of 29 (80.6%) subjects were Caucasian, 3 (8.3%) Asian, 2 (5.6%) Black/African American, and 2 (5.6%) other. The subjects received a total of 4,513 infusions (3,131 for prophylaxis and 1,382 for on-demand).
| MedDRA System Organ Class | Adverse Reaction | Number of ARs | Number of Subjects | Percent of Subjects (N=36) |
|---|---|---|---|---|
| Blood And Lymphatic System Disorders | Anemia | 2 | 2 | 5.6 |
| Gastrointestinal Disorders | Diarrhea | 2 | 2 | 5.6 |
| Nausea | 2 | 2 | 5.6 | |
| Vomiting | 2 | 2 | 5.6 | |
| Investigations | Hepatitis B Surface Antibody Positive | 4 | 4 | 11.1 |
| Musculoskeletal And Connective Tissue Disorders | Hemarthrosis | 5 | 3 | 8.3 |
Table 3 lists the adverse reactions and infusion related adverse events reported in >5% of subjects in any of the three groups (50% Reduced Volume, Increased Rate 4 U/kg/min; 50% Reduced Volume, Increased Rate 10 U/kg/min; Overall 50% Reduced Volume) of the randomized, prospective crossover trial evaluating the tolerability and safety of infusing reduced volume of FEIBA at the standard infusion rate of 2 U/kg/min, and at increased rates of 4 and 10 U/kg/min in a total of 33 treated subjects with congenital Hemophilia A with inhibitors.
| MedDRA System Organ Class | Adverse Reaction | 50% Reduced Volume; Increased Rate 4 U/kg/min (N=30) n (%); m | 50% Reduced Volume; Increased Rate 10 U/kg/min (N=28) n (%); m | Overall 50% Reduced Volume* (N=30) n (%); m |
|
|---|---|---|---|---|---|
| n=Number of subjects, m=number of events | |||||
|
|||||
| Adverse reactions (ARs) | Nervous system disorders | Headache | 0 | 0 | 2 (6.7%); 2 |
| Infusion related AEs | Nervous system disorders | Headache | 0 | 0 | 2 (6.7%); 2 |
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of FEIBA. Because postmarketing reporting of adverse reactions is voluntary and from a population of uncertain size, it is not always possible to reliably estimate the frequency of these reactions or establish a causal relationship to product exposure.
Blood and Lymphatic System Disorders: disseminated intravascular coagulation
Cardiac Disorders: tachycardia, flushing
Respiratory, Thoracic, and Mediastinal Disorders: bronchospasm, wheezing
Gastrointestinal Disorders: abdominal discomfort
Skin and Subcutaneous Tissue Disorders: pruritus
General Disorders and Administration Site Conditions: malaise, feeling hot, injection site pain
Investigations: Fibrin D-dimer increased
7 DRUG INTERACTIONS
7.1 Concomitant Medications
Consider the possibility of thrombotic events when systemic antifibrinolytics such as tranexamic acid and aminocaproic acid are used during treatment with FEIBA. No adequate and well-controlled studies of the combined or sequential use of FEIBA and recombinant factor VIIa antifibrinolytics, or emicizumab have been conducted. Use of antifibrinolytics within approximately 6 to 12 hours after the administration of FEIBA is not recommended.
Clinical experience from an emicizumab clinical trial suggests that a potential drug interaction may exist with emicizumab when FEIBA was used as part of a treatment regimen for breakthrough bleeding6 [see Warnings and Precautions (5.1)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no data with FEIBA use in pregnant women to inform a drug-associated risk. There are no adequate and well-controlled studies in pregnant women. Animal reproduction studies have not been conducted with FEIBA. It is also not known whether FEIBA can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity.
In the U.S. general population, the estimated background risk for major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
There is no information available on the effect of FEIBA on labor and delivery.
8.2 Lactation
Risk Summary
There is no information regarding the presence of FEIBA in human milk, the effect on the breastfed child, or the effects on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for FEIBA and any potential adverse effects on the breastfed child from FEIBA or from the underlying condition.
8.4 Pediatric Use
Safety and efficacy of FEIBA have been evaluated in nine pediatric subjects treated in the routine prophylaxis trial including 4 subjects ≥7 to <12 years of age and 5 subjects ≥12 to <16 years of age. The dosing for all pediatric subjects was based on body weight. A total of 576 infusions were given for the treatment of 223 bleeding episodes (504 infusions for joint bleeding episodes, 72 infusions for muscle and soft tissue bleeding episodes). In 223 (100%) of the episodes, hemostasis was achieved with one or more infusions. Hemostatic efficacy was rated as excellent or good in a majority (96.9%) of the bleeding episodes in both regimens at 24 hours post infusion. The median annualized bleeding episode rate (ABR) for children ≥7 to <12 years of age was 7.7 bleeds per patient per year, as compared to 39 for subjects treated with on-demand therapy [see Clinical Studies (14)].
The safety and efficacy of FEIBA has not been evaluated in neonates.
11 DESCRIPTION
FEIBA (Anti-Inhibitor Coagulant Complex) is a freeze-dried sterile human plasma fraction with factor VIII inhibitor bypassing activity to be reconstituted for intravenous administration. Factor VIII inhibitor bypassing activity is expressed in arbitrary units. One unit of activity is defined as that amount of FEIBA that shortens the aPTT of high titer factor VIII inhibitor reference plasma to 50% of the blank value.
FEIBA contains mainly non-activated factors II, IX, and X and mainly activated factor VII. It contains approximately equal unitages of factor VIII inhibitor bypassing activity and prothrombin complex factors. In addition, the preparation contains 1-6 units of factor VIII coagulant antigen (FVIII C:Ag) per mL. The product contains traces of factors of the kinin generating system. It contains no heparin. Reconstituted FEIBA contains 4 mg of trisodium citrate and 8 mg of sodium chloride per mL.
FEIBA is manufactured from large pools of human plasma. Screening against potentially infectious agents begins with the donor selection process and continues throughout plasma collection and plasma preparation. Each individual plasma donation used in the manufacture of FEIBA is collected at FDA approved blood establishments and is tested by FDA licensed serological tests for Hepatitis B Surface Antigen (HBsAg), and for antibodies to Human Immunodeficiency Virus (HIV-1/HIV-2) and Hepatitis C Virus (HCV) Mini-pools of the plasma are tested and found negative for the presence of HIV-1 and HCV by FDA licensed Nucleic Acid Testing (NAT).
To reduce the risk of viral transmission, the manufacturing process of FEIBA includes two dedicated and independent virus removal/inactivation steps namely 35 nm nanofiltration and a vapor heat treatment process. In addition, the DEAE-Sephadex adsorption contributes to the virus safety profile of FEIBA.
In vitro spiking studies have been used to validate the capability of the manufacturing process to remove and inactivate viruses. Table 4 summarizes the results of the viral clearance studies for FEIBA.
| Virus Type | Enveloped RNA | Enveloped DNA | Non-enveloped RNA | Non-enveloped DNA | |||
|---|---|---|---|---|---|---|---|
| Virus Family | Retroviridae | Flaviviridae | Herpesviridae | Picornaviridae | Parvoviridae | ||
|
|||||||
| Virus* | HIV-1 | BVDV | WNV | PRV | HAV | B19V† | MMV |
| DEAE Sephadex Adsorption | 3.2 | 1.8 | ND | 2.5 | 1.5 | 1.7 | 1.2 |
| 35 nm Nanofiltration | >5.3 | 2.1 | 4.7 | >5.7 | 2.6 | 0.2‡ | 1.0 |
| Vapor-Heat Treatment | >5.9 | >5.6 | >8.1 | >6.7 | >5.2 | 3.5 | 0.9‡ |
| Overall virus reduction factor (log10) | >14.4 | >9.5 | >12.8 | >14.9 | >9.3 | 5.2 | 2.2 |
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of FEIBA is still the subject of scientific discussion. FEIBA contains multiple components, mainly non-activated factors II, IX, X and mainly activated factor VII. These factors can interact with plasma coagulation factors and platelets to increase the impaired thrombin generation of hemophilia patients with inhibitors, leading to hemostasis.
12.2 Pharmacodynamics
Laboratory assessment of coagulation does not necessarily correlate with or predict the hemostatic effectiveness of FEIBA. Factor VIII inhibitor bypassing activity of FEIBA has been demonstrated in vitro as well as in vivo. FEIBA can shorten the activated partial thromboplastin time (aPTT) and increase the thrombin generation in plasma containing factor VIII inhibitor.4,5
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies to evaluate the carcinogenic potential of FEIBA or studies to determine the genotoxicity or the effect of FEIBA on fertility have not been performed. An assessment of the carcinogenic potential of FEIBA was completed to demonstrate minimal carcinogenic risk from product use.
14 CLINICAL STUDIES
Control and Prevention of Bleeding Episodes
The efficacy of FEIBA in the treatment of bleeding episodes has been demonstrated by two prospective clinical trials.1,2
The first trial was a multicenter, randomized, double-blind trial comparing the effect of FEIBA and a non-activated prothrombin complex concentrate in 15 subjects with hemophilia A and inhibitors to factor VIII. The inclusion criteria were history of high titer inhibitors, high responder status, more than 1 bleeding episode per month in the prior year and no signs of liver failure. A total of 150 bleeding episodes including 117 joint, 20 musculoskeletal and 4 mucocutaneous bleeds, were treated. A single dose of 88 units per kg of body weight was used uniformly for treatments with FEIBA. A second treatment was allowed for muscle bleeds after 12 hours and 6 hours after mucocutaneous bleeds, if necessary.
Subjects and investigators were asked to rate hemostatic efficacy based on a scale of effective, partially effective, not effective or not sure. The criteria for evaluation of the effectiveness were severity of pain, subjective improvement, circumference of muscle or joint, restriction of joint mobility, cessation of open bleeding, start of rebleeding and quantity and nature of analgesics. FEIBA was effective in 41% and partly effective in 25% of episodes (i.e. combined effectiveness of 66%), while prothrombin complex concentrate was rated effective in 25% and partly effective in 21% of episodes (i.e., combined effectiveness of 46%).
The second trial with FEIBA was a multicenter randomized, prospective trial. This trial was conducted in 44 hemophilia A subjects with inhibitors, 3 hemophilia B subjects with inhibitors and 2 acquired factor VIII inhibitor subjects. It was designed to evaluate the efficacy of FEIBA in the treatment of joint, mucous membrane, musculocutaneous and emergency bleeding episodes such as central nervous system hemorrhages and surgical bleedings. The inclusion criteria used were age >4 years, history of inhibitor titer ≥4 Bethesda Units (BU) and without chronic liver disease. Subjects were excluded if they had a history of thromboembolic events or allergic reactions to FEIBA.
Forty-nine (49) subjects with inhibitor titers of greater than 5 BU were enrolled from nine co-operating hemophilia centers. Subjects were treated with 50 units per kg of body weight, repeated at 12-hour intervals (6-hour intervals in mucous membrane bleedings), if necessary. A total of 489 infusions were given for the treatment of 165 bleeding episodes (102 joint, 33 muscle and soft tissue, 20 mucous membrane, and 10 emergency bleeds, including 3 central nervous system bleeds and 4 surgical procedures). Bleeding was controlled in 153 episodes (93%). In 130 (78%) of the episodes, hemostasis was achieved with one or more infusions within 36 hours. Of these, 36% were controlled with one infusion within 12 hours. An additional 14% of episodes responded after more than 36 hours.
Routine Prophylaxis
In a multicenter, open-label, prospective, randomized clinical trial comparing subjects receiving FEIBA for prophylaxis with subjects receiving FEIBA for on-demand treatment, 36 hemophilia A and B subjects with inhibitors to factor VIII or IX were analyzed in the intent-to-treat analysis. Study population included 29 (80.6%) Caucasian, 3 (8.3%) Asian, 2 (5.6%) Black/African American, and 2 (5.6%) other. Inclusion criteria were subjects with a history of high titer inhibitors or low titer refractory to increased factor VIII or IX dosing, age range between 4 and 65, and subjects receiving bypassing agents with ≥12 bleeds in the 12 months prior to trial entry. Subjects with a history of thromboembolic events, symptomatic liver disease, or a platelet count <100,000 per mL, and those receiving immune tolerance induction or routine prophylaxis were excluded.
Subjects were randomized to receive 12 months of prophylactic or on-demand treatment with FEIBA. Seventeen subjects randomized to the prophylaxis arm received 85 units per kg of FEIBA every other day. Nineteen subjects randomized to the on-demand arm received FEIBA for the treatment of acute bleeding episodes per the dose and dosing regimen recommended. Target joints were defined as ≥4 bleeding episodes within 6 months. In this trial, ankles, knees, elbows and hips were target joint locations. Preexisting target joints were not considered as new target joints.
Hemostatic efficacy for treatment of acute bleeds was evaluated at 6 and 24 hours according to a pre-specified four-point scale of excellent, good, fair, or none. An evaluation of "none" was considered a treatment failure. The criteria for evaluation of the effectiveness were relief of pain, cessation of bleeding, and number of infusions required to treat a bleed.
A total of 825 bleeding episodes were reported including 196 that occurred during prophylaxis and 629 that occurred during on-demand therapy. A majority (78%) of the 794 bleeding episodes that were rated for efficacy were treated with 1 or 2 infusions. Hemostatic efficacy was rated as excellent or good for 74% of bleeding episodes rated at 6 hours post infusion and for 87% of the bleeding episodes at 24 hour post infusion. A total of 19 (2.4%) bleeds were rated as "none" at 6 hours post infusion; 1 bleed (0.1%) was rated "none" at 24 hours.
Hemostatic efficacy for routine prophylaxis was evaluated against subjects who received on-demand therapy.
The overall median annual bleed rate (ABR) for the on-demand arm was 28.7 compared to 7.9 for the prophylaxis arm, which represents a 72% reduction in median ABR with prophylaxis. When analyzed by site (e.g. joint, non-joint) and cause of bleed (e.g. spontaneous, traumatic), prophylactic treatment with FEIBA resulted in a greater than 50% reduction in ABR. There were fewer subjects in the prophylaxis arm who developed new target joints (7 new target joints in 5 subjects treated with prophylaxis compared to 23 new target joints in 11 subjects in the on-demand arm). Target joints developed in two subjects in the on-demand arm and three in the prophylaxis arm who did not have reported target joints at trial enrollment. A total of 3 of 17 (18%) subjects had no bleeding episodes on prophylaxis. In the on-demand arm, all subjects experienced a bleeding episode.
ABR by age category between on-demand and prophylaxis regimens is provided in Table 5. One adolescent subject on prophylaxis had a higher rate of bleeding possibly due to increased physical activity after study enrollment.
| Age Category | On-Demand: Number of Subjects | On-Demand: ABR Median | Prophylaxis: Number of Subjects | Prophylaxis: ABR Median |
|---|---|---|---|---|
| Children (≥7 to <12 years old) | 2 | 39.3 | 2 | 7.7 |
| Adolescent (≥12 to <16 years old) | 2 | 30.9 | 3 | 27.5 |
| Adult (≥16 years old) | 15 | 23.9 | 12 | 6.9 |
15 REFERENCES
- Sjamsoedin LJ, Heijnen L, Mauser-Bunschoten EP, van Geijlswijk JL, van Houwelingen H, van Asten P, Sixma JJ. The effect of Activated Prothrombin-Complex Concentrate (FEIBA) on joint and muscle bleeding in patients with Hemophilia A and antibodies to Factor VIII. N Engl J Med. 1981;305(13): 717-721.
- Hilgartner MW, Knatterud GL. The use of Factor-Eight-Inhibitor-By-Passing-Activity (FEIBA IMMUNO) product for treatment of bleeding episodes in Hemophiliacs with inhibitors. Blood. 1983;61(1): 36-40.
- Antunes SV, Tangada S, Stasyshyn O, Mamonov V, Phillips J, Guzman-Becerra N, Grigorian A, Ewenstein B, Wong WY. Randomized comparison of prophylaxis and on-demand regimens with FEIBA NF in the treatment of haemophilia A and B with inhibitors. Haemophilia. 2013; DOI 10.1111/hae.12246.
- Turecek PL, Varadi K, Gritsch H, Auer W, Pichler L, Eder G, Schwarz HP. Factor Xa and prothrombin: mechanism of action of FEIBA. Vox Sanguinis 1999;77 Suppl 1:72-79.
- Turecek PL, Varadi K, Gritch H, Schwarz HP. FEIBA: Mode of action Haemophilia 2004;10: Suppl. 2:3-9
- Oldenburg et al. Emicizumab Prophylaxis in Hemophilia A with Inhibitors. N Engl J Med 2017:377:809-818.
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
FEIBA is available in single-dose vials in the following nominal dosage strengths:
| Nominal Strength | Color Code | Factor VIII Potency Range | Kit NDC | Sterile Water Volume |
|---|---|---|---|---|
| 500 units | Orange | 350-650 units per vial | 64193-426-02 | 10 mL |
| 1000 units | Green | 700-1300 units per vial | 64193-424-02 | 20 mL |
| 2500 units | Purple | 1750-3250 units per vial | 64193-425-02 | 50 mL |
The number of units of factor VIII inhibitor bypassing activity is stated on the label of each vial.
FEIBA is packaged with a suitable volume (10 mL, 20 mL, or 50 mL) of Sterile Water for Injection, U.S.P., one BAXJECT II Hi-Flow Needleless Transfer Device, and one Package Insert.
Not made with natural rubber latex.
17 PATIENT COUNSELING INFORMATION
Inform patients:
- of the signs and symptoms of thrombosis, such as chest pain or pressure, shortness of breath, altered consciousness, vision, or speech, limb or abdomen swelling and/or pain. Advise patients to seek immediate medical attention if any of these symptoms occur.
- of the signs and symptoms of hypersensitivity reactions, such as urticaria, angioedema, gastrointestinal manifestations, bronchospasm, and hypotension. Advise patients to discontinue use of the product if these symptoms occur and seek immediate emergency treatment.
- that because FEIBA is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and theoretically, the Creutzfeldt-Jakob disease (CJD) agent.
- if they are on emicizumab prophylaxis therapy and need FEIBA to treat a breakthrough bleeding episode then they must be monitored by their hemophilia treating physician, preferably at the hemophilia treatment center (HTC).
- to report any adverse reactions or problems following FEIBA administration to their hemophilia treating physician.
To enroll in the confidential, Industry-wide Patient Notification System, call 1-888-873-2838.
FEIBA® is a registered trademark of Baxalta Incorporated, a Takeda company.
TAKEDA® and the TAKEDA Logo® are registered trademarks of Takeda Pharmaceutical Company Limited.
Takeda Pharmaceuticals U.S.A., Inc.
Lexington, MA 02421
U.S. License No. 1898
PRINCIPAL DISPLAY PANEL - Kit Carton - NDC 64193-424-02
20 mL size, dried
NDC 64193-424-02
Anti-Inhibitor Coagulant Complex
FEIBA
Nanofiltered & Vapor Heated
For Intravenous Use After Reconstitution
Contains no preservative
Dosage and Administration: See accompanying package insert.
Storage: 36°F to 77°F (2°C to 25°C). Store in the original package in order to protect from light.
Reconstitution: Use 20 mL sWFI, U.S.P.;Do not refrigerate after reconstitution.; Use within 3 hours of
reconstitution.
Do Not Freeze
Rx Only
Takeda
Includes
BAXJECT II Hi-Flow
Needleless Transfer Device

PRINCIPAL DISPLAY PANEL - 20 mL Vial Label
20 mL size, dried
NDC 64193-324-01
Anti-Inhibitor Coagulant Complex
FEIBA
Nanofiltered & Vapor Heated
For Intravenous Use After Reconstitution
Contains no preservative
Dosage & Administration: See accompanying package insert.
Storage: 36°F to 77°F (2°C to 25°C).
Reconstitution: Use 20 mL sWFI, U.S.P.; Do not refrigerate
after reconstitution.; Use within 3 hours of reconstitution.
Rx only
0754401

PRINCIPAL DISPLAY PANEL - Kit Carton - NDC 64193-425-02
Single-dose vial
NDC 64193-425-02
Anti-Inhibitor Coagulant Complex
FEIBA
Nanofiltered & Vapor Heated
For Intravenous Use After Reconstitution
Contains no preservative
Dosage and Administration: See accompanying package insert.
Storage: 36°F to 77°F (2°C to 25°C).
Store in the original package in order to protect from light.
Reconstitution: Use 50 mL sWFI, U.S.P.; Do not refrigerate after reconstitution.; Use within 3 hours of
reconstitution.
Do Not Freeze
Rx Only
Takeda
Includes
BAXJECT II Hi-Flow
Needleless Transfer Device

PRINCIPAL DISPLAY PANEL - 50 mL Vial Label
50 mL size, dried
NDC 64193-325-01
Anti-Inhibitor Coagulant Complex
FEIBA
Nanofiltered & Vapor Heated
For Intravenous Use After Reconstitution
Contains no preservative
Dosage & Administration: See accompanying package insert.
Storage: 36°F to 77°F (2°C to 25°C).
Reconstitution: Use 50 mL sWFI, U.S.P.; Do not refrigerate
after reconstitution.; Use within 3 hours of reconstitution.
Rx only
0754404

PRINCIPAL DISPLAY PANEL - Kit Carton - NDC 64193-426-02
Single-dose vial
NDC 64193-426-02
Anti-Inhibitor
Coagulant Complex
FEIBA
Nanofiltered & Vapor Heated
For Intravenous Use After Reconstitution
Contains no preservative
Dosage and Administration: See accompanying package insert.
Storage: 36°F to 77°F (2°C to 25°C). Store in the original package in order to protect
from light.
Reconstitution: Use 10 mL sWFI, U.S.P.; Do not refrigerate after reconstitution.; Use within
3 hours of reconstitution.
Do Not Freeze
Rx Only
Takeda
Includes
BAXJECT II Hi-Flow
Needleless Transfer Device

PRINCIPAL DISPLAY PANEL - 10 mL Vial Label
Single-dose vial
NDC 64193-326-01
Anti-Inhibitor Coagulant Complex
FEIBA
Nanofiltered & Vapor Heated
For Intravenous Use After Reconstitution
No preservative
Dosage & Administration: See accompanying package insert.
Storage: 36°F to 77°F (2°C to 25°C).
Reconstitution: Use 10 mL sWFI, U.S.P.; Do not refrigerate after
reconstitution.; Use within 3 hours of reconstitution.
Rx only
Takeda
0754406
