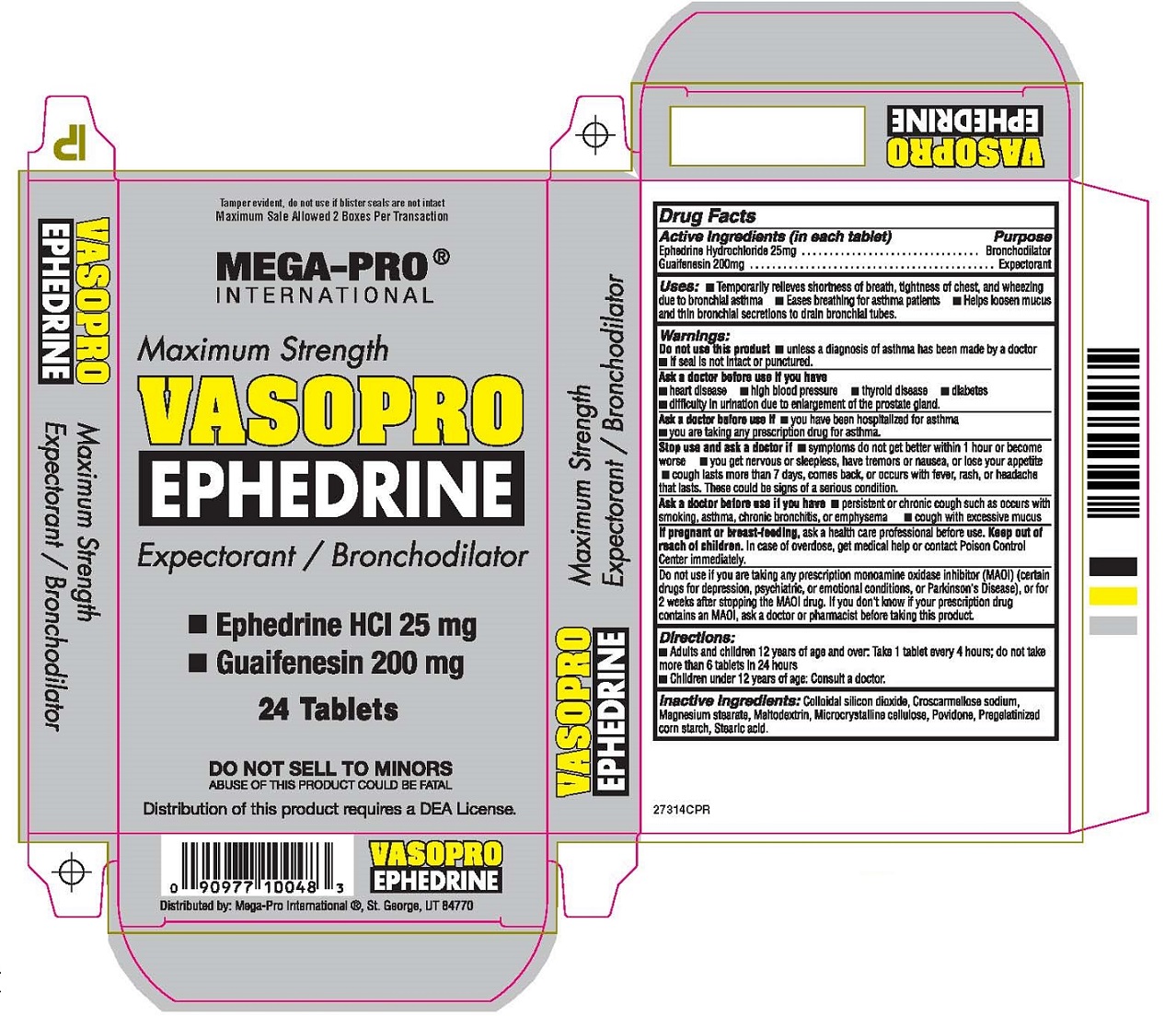
Keep out of reach of children. In case of overdose, get medical help or contact Poison Control Center immediately.
Uses: • Temporarily relieves shortness of breath, tightness of chest, and wheezing due to bronchial asthma
• Eases breathing for asthma patients • Helps loosen mucus and thin bronchial secretions to drain bronchial tubes.
Warnings:
Do not use this product • unless a diagnosis of asthma has been made by a doctor
• If seal is not intact or punctured.
Ask a doctor before use if you have
• heart disease • high blood pressure • thyroid disease • diabetes
• difficulty in urination due to enlargement of the prostate gland.
Ask a doctor before use if • you have been hospitalized for asthma
• you are taking any prescription drug for asthma.
Stop use and ask a doctor if • symptoms do not get better within 1 hour or become worse
• you get nervous or sleepless, have tremors or nausea, or lose your appetite
• cough lasts more than 7 days, comes back, or occurs with fever, rash, or headache that lasts.
These could be signs of a serious condition.
Ask a doctor before use if you have • persistent or chronic cough such as occurs with smoking, asthma,
chronic bronchitis, or emphysema • cough with excessive mucus
If pregnant or breast-feeding, ask a health care professional before use.
Do not use if you are taking any prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression,
psychiatric, or emotional conditions, or Parkinson’s Disease), or for 2 weeks after stopping the MAOI drug.
If you don’t know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Directions:
• Adults and children 12 years of age and over. Take 1 tablet every 4 hours: do not take more than 6 tablets in 24 hours
• Children under 12 years of age: Consult a doctor.
Inactive Ingredients: Colloidal silicon dioxide, Croscarmellose sodium, Magnesium stearate, Maltodextrin, Microcrystalline cellulose
Povidone, Pregelatinized corn starch, Stearic acid.
Tamper evident, do not use if blister seals are not intact
Maximum Sale Allowed 2 Boxes Per Transaction
MEGA-PRO®
INTERNATIONAL
Maximum Strength
VASOPRO
EPHEDRINE
Expectorant / Bronchodilator
24 Tablets
DO NOT SELL TO MINORS
ABUSE OF THIS PRODUCT COULD BE FATAL
Distribution of this product requires a DEA License.
Distributed by: Mega-Pro International®, St. George, UT 84770