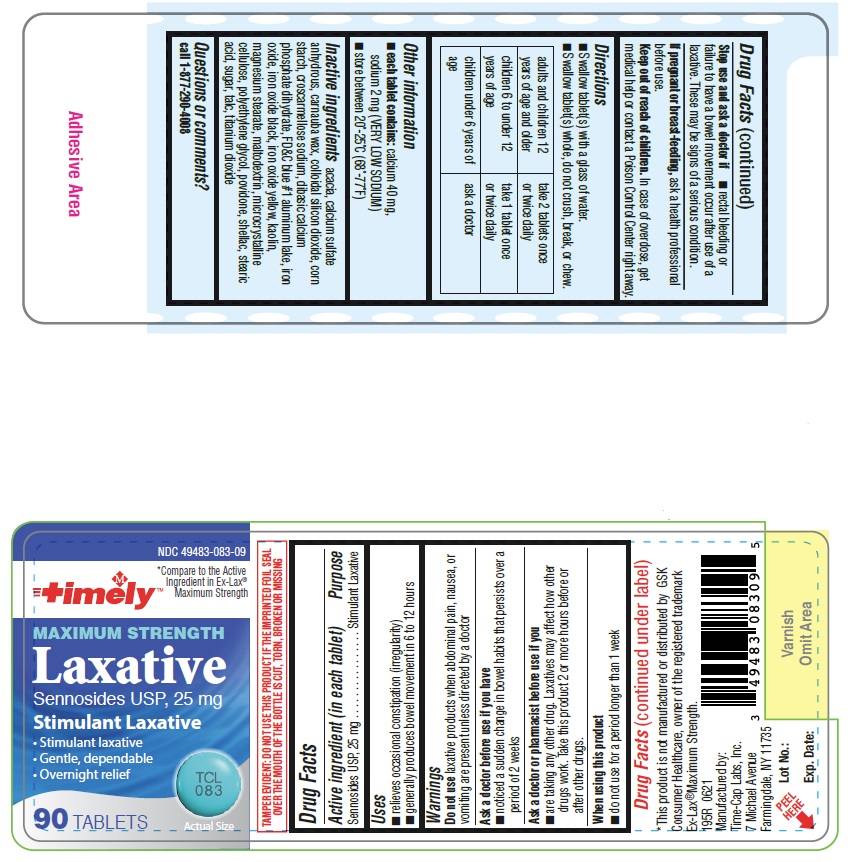
INACTIVE INGREDIENTS: acacia, calcium sulfate anhydrous, carnauba wax, colloidal silicon dioxide, corn starch, croscarmellose sodium, dibasic calcium phosphate dihydrate, FD&C blue#1, iron oxide, iron oxide black, iron oxide yellow, kaolin, magnesium stearate, maltodextrin, microcrystalline cellulose, polyethylene glycol, povidone, shellac, stearic acid, sugar, talc, titanium dioxide
Directions:
Take preferably at bedtime or as directed by a doctor. If you do not have a comfortable bowel movement by the second day, increase dose by one tablet (not to exceed maximum dosage) or decrease dose until you are comfortable.
Adults and children 12 years and over - starting dosage: 2 tablets once a day maximum dosage: 4 tablets twice a day
Children 6 to under 12 years - starting dosage: 1 tablet once a day maximum dosage: 2 tablets twice a day
Children 6 to under 12 years - starting dosage: 1/2 tablet once a day maximum dosage: 1 tablet twice a day
Children under 2 years - Ask a doctor
Uses:
Relieves occasional constipation (irregularity); generally causes bowel movement in 6-12 hours
WARNINGS:
Do not use this product
If you are presently taking mineral oil, unless directed by a doctor
Laxative products for longer than 1 week unless directed by a doctor