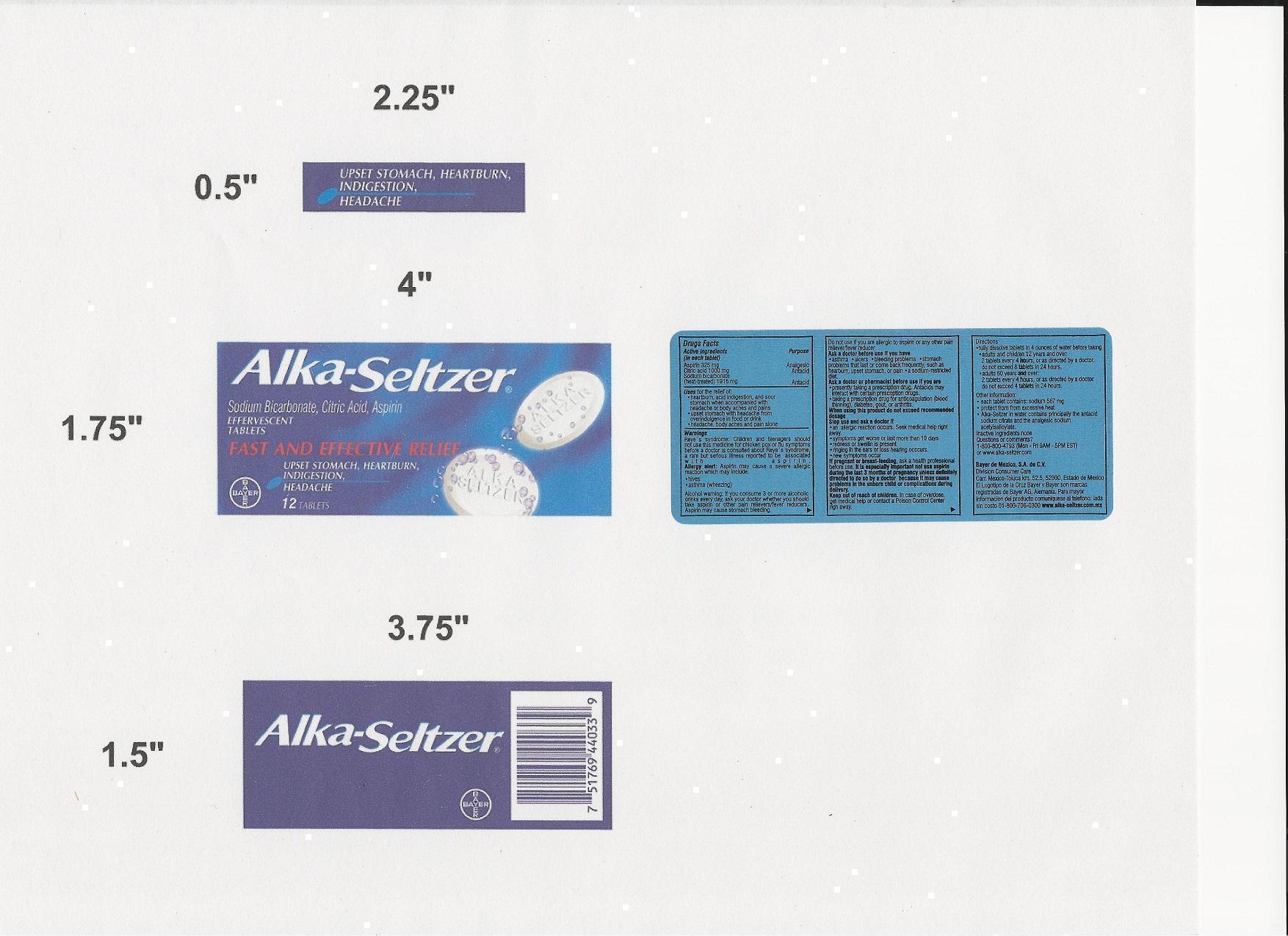
Active Ingredients
(in each tablet)
Aspirin 325 mg
Citric acid 1000 mg
Sodium Bicarbonate (heat treated) 1916 mg
Keep out of reach of children
In case of overdose, get medical help or contact a Poison Control Center right away.
Uses for the relief of:
- heartburn, acid indigestion, and sour stomach when accompanied with headache or body aches and pains
- upset stomach with headache from overindulgence in food or drink
- headache, body aches, and pain alone
Warnings
Reye's syndrome: Children and teenagers should not use this medicine for chicken pox or flu symptoms before a doctor is consulted about Reye's syndrome, a rare but serious illness reported to be associated with aspirin.
Allergy alert: Aspirin may cause a severe allergic reaction which may include:
- hives
- asthma (wheezing)
Alcohol warning: If you consume 3 or more alcoholic drinks every day, ask your doctor whether you should take aspirin or other pain relievers/fever reducers. Aspirin may cause stomach bleeding.
Directions
- fully dissolve 2 tablets in 4 ounces of water before taking
- adults and children 12 years and over: 2 tablets every 4 hours, or as directed by a doctor do not exceed 8 tablets in 24 hours
- adults 60 years and over 2 tablets every 4 hours, or as directed by a doctor do not exceed 4 tablets in 24 hours
- children under 12 years consult a doctor
Other information
- each tablet contains: sodium 567 mg
- store at room temperature. Avoid excessive heat.
- Alka-Seltzer in water contains principally the antacid sodium citrate and the analgesic sodium acetylsalicylate