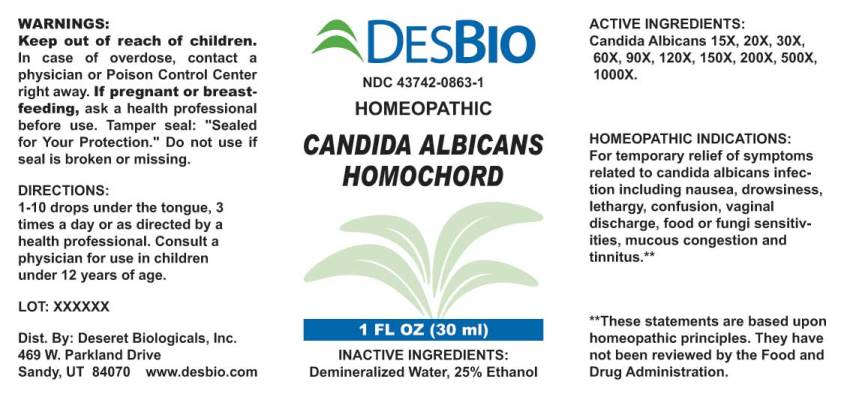
HOMEOPATHIC INDICATIONS:
For temporary relief of symptoms related to candida albicans infection including nausea, drowsiness, lethargy, confusion, vaginal discharge, food or fungi sensitivities, petrochemicals, mucous congestion and tinnitus.**
**These statements are based upon traditional homeopathic principles. They have not been reviewed by the Food and Drug Administration.
WARNINGS:
Keep out of reach of children. In case of overdose, contact physician or a Poison Control Center right away.
If pregnant or breast-feeding, ask a health professional before use.
Tamper seal: "Sealed for Your Protection." Do not use if seal is broken or missing.
KEEP OUT OF REACH OF CHILDREN:
Keep out of reach of children. In case of overdose, contact physician or a Poison Control Center right away.
DIRECTIONS:
1-10 drops under the tongue, 3 times a day or as directed by a health professional. Consult a physician for use in children under 12 years of age.
HOMEOPATHIC INDICATIONS:
For temporary relief of symptoms related to candida albicans infection including nausea, drowsiness, lethargy, confusion, vaginal discharge, food or fungi sensitivities, petrochemicals, mucous congestion and tinnitus.**
**These statements are based upon traditional homeopathic principles. They have not been reviewed by the Food and Drug Administration.