FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
INTELENCE®1, in combination with other antiretroviral agents, is indicated for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in antiretroviral treatment-experienced patients ages 6 years and older, who have evidence of viral replication and HIV-1 strains resistant to a non-nucleoside reverse transcriptase inhibitor (NNRTI) and other antiretroviral agents.
The indication for adult use is based on Week 48 analyses from 2 randomized, double-blind, placebo-controlled trials of INTELENCE®. Both studies were conducted in clinically advanced, 3-class antiretroviral (NNRTI, N[t]RTI, PI) treatment-experienced adults. The indication for pediatric use is based on 24-week analyses of a single-arm, Phase 2 trial in antiretroviral treatment-experienced pediatric subjects 6 years to less than 18 years of age [see Use in Specific Populations (8.4)].
In treatment-experienced adult and pediatric patients, the following points should be considered when initiating therapy with INTELENCE®:
- Treatment history and resistance testing should guide the use of INTELENCE® due to concerns for potential cross-resistance [see Microbiology (12.4) and Clinical Studies (14)].
- In patients who have experienced virologic failure on an NNRTI-containing regimen, do not use INTELENCE® in combination with only N[t]RTIs [see Clinical Studies (14)].
- The use of other active antiretroviral agents with INTELENCE® is associated with an increased likelihood of treatment response.
- The safety and efficacy of INTELENCE® have not been established in pediatric patients less than 6 years of age or in treatment-naïve adult or pediatric patients.
- 1
- Registered trademark of Tibotec Pharmaceuticals
2 DOSAGE AND ADMINISTRATION
2.1 Adult Patients
The recommended oral dose of INTELENCE® tablets is 200 mg (one 200 mg tablet or two 100 mg tablets) taken twice daily following a meal [see Clinical Pharmacology (12.3)]. The type of food does not affect the exposure to etravirine.
2.2 Pediatric Patients (6 years to less than 18 years of age)
The recommended dose of INTELENCE® for pediatric patients 6 years to less than 18 years of age and weighing at least 16 kg is based on body weight (see table below) not exceeding the recommended adult dose. INTELENCE tablet(s) should be taken orally, following a meal [see Clinical Pharmacology (12.3)]. The type of food does not affect the exposure to etravirine.
| Weight kilograms (kg) | Dose |
|---|---|
| greater than or equal to 16 kg to less than 20 kg | 100 mg twice daily |
| greater than or equal to 20 kg to less than 25 kg | 125 mg twice daily |
| greater than or equal to 25 kg to less than 30 kg | 150 mg twice daily |
| greater than or equal to 30 kg | 200 mg twice daily |
The safety and efficacy of INTELENCE® have not been established in children less than 6 years of age [see Clinical Pharmacology (12.3)].
Healthcare professionals should pay special attention to the accurate dose selection of INTELENCE®, the transcription of the medication order, the dispensing information and the dosing instructions to minimize the risk of medication errors, overdosing, and underdosing.
2.3 Method of Administration
Patients should be instructed to swallow the INTELENCE® tablet(s) whole with a liquid such as water. Patients who are unable to swallow the INTELENCE® tablet(s) whole may disperse the tablet(s) in a glass of water. The patient should be instructed to do the following:
- place the tablet(s) in 5 ml (1 teaspoon) of water, or at least enough liquid to cover the medication,
- stir well until the water looks milky,
- if desired, add more water or alternatively orange juice or milk (patients should not place the tablets in orange juice or milk without first adding water). The use of grapefruit juice or warm (greater than 40°C) or carbonated beverages should be avoided.
- drink it immediately,
- rinse the glass several times with water, orange juice, or milk and completely swallow the rinse each time to make sure the patient takes the entire dose.
3 DOSAGE FORMS AND STRENGTHS
3.1 INTELENCE® 25 mg Tablets
INTELENCE® 25 mg tablets are supplied as white to off-white, oval, scored tablets debossed with "TMC" on one side.
5 WARNINGS AND PRECAUTIONS
5.1 Severe Skin and Hypersensitivity Reactions
Severe, potentially life-threatening, and fatal skin reactions have been reported. These include cases of Stevens-Johnson syndrome, toxic epidermal necrolysis and erythema multiforme. Hypersensitivity reactions have also been reported and were characterized by rash, constitutional findings, and sometimes organ dysfunction, including hepatic failure. In Phase 3 clinical trials, Grade 3 and 4 rashes were reported in 1.3% of subjects receiving INTELENCE® compared to 0.2% of placebo subjects. A total of 2.2% of HIV-1-infected subjects receiving INTELENCE® discontinued from Phase 3 trials due to rash [see Adverse Reactions (6)]. Rash occurred most commonly during the first 6 weeks of therapy.
Discontinue INTELENCE® immediately if signs or symptoms of severe skin reactions or hypersensitivity reactions develop (including, but not limited to, severe rash or rash accompanied by fever, general malaise, fatigue, muscle or joint aches, blisters, oral lesions, conjunctivitis, facial edema, hepatitis, eosinophilia, angioedema). Clinical status including liver transaminases should be monitored and appropriate therapy initiated. Delay in stopping INTELENCE® treatment after the onset of severe rash may result in a life-threatening reaction.
5.2 Fat Redistribution
Redistribution/accumulation of body fat, including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and "cushingoid appearance" have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
5.3 Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including INTELENCE®. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jiroveci pneumonia (PCP) or tuberculosis), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves' disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable, and can occur many months after initiation of treatment.
6 ADVERSE REACTIONS
The following adverse reactions are described in greater detail in other sections:
- Severe skin and hypersensitivity reactions [see Warnings and Precautions (5.1)].
6.1 Clinical Trials Experience: Adults
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety assessment is based on all data from 1203 subjects in the Phase 3 placebo-controlled trials, TMC125-C206 and TMC125-C216, conducted in antiretroviral treatment-experienced HIV-1-infected adult subjects, 599 of whom received INTELENCE® (200 mg twice daily). In these pooled trials, the median exposure for subjects in the INTELENCE® arm and placebo arm was 52.3 and 51.0 weeks, respectively. Discontinuations due to adverse drug reactions (ADRs) were 5.2% in the INTELENCE® arm and 2.6% in the placebo arm.
The most frequently reported ADR at least Grade 2 in severity was rash (10.0%). Stevens-Johnson syndrome, drug hypersensitivity reaction and erythema multiforme were reported in less than 0.1% of subjects during clinical development with INTELENCE® [see Warnings and Precautions (5.1)]. A total of 2.2% of HIV-1-infected subjects in Phase 3 trials receiving INTELENCE® discontinued due to rash. In general, in clinical trials, rash was mild to moderate, occurred primarily in the second week of therapy, and was infrequent after Week 4. Rash generally resolved within 1 to 2 weeks on continued therapy. The incidence of rash was higher in women compared to men in the INTELENCE® arm in the Phase 3 trials. Patients with a history of NNRTI-related rash did not appear to be at increased risk for the development of INTELENCE®-related rash compared to patients without a history of NNRTI-related rash.
Common Adverse Reactions
Clinical ADRs of moderate intensity or greater (greater than or equal to Grade 2) and reported in at least 2% of subjects treated with INTELENCE® and occurring at a higher rate compared to placebo (excess of 1%) are presented in Table 1. Laboratory abnormalities considered ADRs are included in Table 2.
| System Organ Class, Preferred Term, % | Pooled TMC125-C206 and TMC125-C216 Trials | |
|---|---|---|
| INTELENCE® + BR N=599 | Placebo + BR N=604 |
|
| N=total number of subjects per treatment group, BR=background regimen | ||
| Nervous System Disorders | ||
| Peripheral neuropathy | 4% | 2% |
| Skin and Subcutaneous Tissue Disorders | ||
| Rash | 10% | 3% |
Less Common Adverse Reactions
Treatment-emergent ADRs occurring in less than 2% of subjects (599 subjects) receiving INTELENCE® and of at least moderate intensity (greater than or equal to Grade 2) are listed below by body system:
Cardiac Disorders: myocardial infarction, angina pectoris, atrial fibrillation
Ear and Labyrinth Disorders: vertigo
Eye Disorders: blurred vision
Gastrointestinal Disorders: gastroesophageal reflux disease, flatulence, gastritis, abdominal distension, pancreatitis, constipation, dry mouth, hematemesis, retching, stomatitis
General Disorders and Administration Site Conditions: sluggishness
Hematologic Disorders: hemolytic anemia
Hepatobiliary Disorders: hepatic failure, hepatomegaly, cytolytic hepatitis, hepatic steatosis, hepatitis
Immune System Disorders: drug hypersensitivity, immune reconstitution syndrome
Metabolism and Nutrition Disorders: diabetes mellitus, anorexia, dyslipidemia
Nervous System Disorders: paraesthesia, somnolence, convulsion, hypoesthesia, amnesia, syncope, disturbance in attention, hypersomnia, tremor
Psychiatric Disorders: anxiety, sleep disorders, abnormal dreams, confusional state, disorientation, nervousness, nightmares
Renal and Urinary Disorders: acute renal failure
Reproductive System and Breast Disorders: gynecomastia
Respiratory,Thoracic and Mediastinal Disorders: exertional dyspnea, bronchospasm
Skin and Subcutaneous Tissue Disorders: night sweats, lipohypertrophy, prurigo, hyperhidrosis, dry skin, swelling face
Additional ADRs of at least moderate intensity observed in other trials were acquired lipodystrophy, angioneurotic edema, erythema multiforme and haemorrhagic stroke, each reported in no more than 0.5% of subjects.
Laboratory Abnormalities in Treatment-Experienced Patients
Selected Grade 2 to Grade 4 laboratory abnormalities that represent a worsening from baseline observed in adult subjects treated with INTELENCE® are presented in Table 2.
| Pooled TMC125-C206 and TMC125-C216 Trials | |||
|---|---|---|---|
| Laboratory Parameter Preferred Term, % | DAIDS Toxicity Range | INTELENCE® + BR N=599 | Placebo + BR N=604 |
| ULN=Upper Limit of Normal, BR=background regimen | |||
| GENERAL BIOCHEMISTRY | |||
| Pancreatic amylase | |||
| Grade 2 | > 1.5–2 × ULN | 7% | 8% |
| Grade 3 | > 2–5 × ULN | 7% | 8% |
| Grade 4 | > 5 × ULN | 2% | 1% |
| Lipase | |||
| Grade 2 | > 1.5–3 × ULN | 4% | 6% |
| Grade 3 | > 3–5 × ULN | 2% | 2% |
| Grade 4 | > 5×ULN | 1% | < 1% |
| Creatinine | |||
| Grade 2 | > 1.4–1.8 × ULN | 6% | 5% |
| Grade 3 | > 1.9–3.4 × ULN | 2% | 1% |
| Grade 4 | > 3.4 × ULN | 0% | < 1% |
| HEMATOLOGY | |||
| Decreased hemoglobin | |||
| Grade 2 | 90–99 g/L | 2% | 4% |
| Grade 3 | 70–89 g/L | < 1% | < 1% |
| Grade 4 | < 70 g/L | < 1% | < 1% |
| White blood cell count | |||
| Grade 2 | 1,500–1,999/mm3 | 2% | 3% |
| Grade 3 | 1,000–1,499/mm3 | 1% | 4% |
| Grade 4 | < 1,000/mm3 | 1% | < 1% |
| Neutrophils | |||
| Grade 2 | 750–999/mm3 | 5% | 6% |
| Grade 3 | 500–749/mm3 | 4% | 4% |
| Grade 4 | < 500/mm3 | 2% | 3% |
| Platelet count | |||
| Grade 2 | 50,000–99,999/mm3 | 3% | 5% |
| Grade 3 | 25,000–49,999/mm3 | 1% | 1% |
| Grade 4 | < 25,000/mm3 | < 1% | < 1% |
| LIPIDS AND GLUCOSE | |||
| Total cholesterol | |||
| Grade 2 | > 6.20–7.77 mmol/L 240–300 mg/dL | 20% | 17% |
| Grade 3 | > 7.77 mmol/L > 300 mg/dL | 8% | 5% |
| Low density lipoprotein | |||
| Grade 2 | 4.13–4.9 mmol/L 160–190 mg/dL | 13% | 12% |
| Grade 3 | > 4.9 mmol/L > 190 mg/dL | 7% | 7% |
| Triglycerides | |||
| Grade 2 | 5.65–8.48 mmol/L 500 –750 mg/dL | 9% | 7% |
| Grade 3 | 8.49–13.56 mmol/L 751 – 1200 mg/dL | 6% | 4% |
| Grade 4 | > 13.56 mmol/L > 1200 mg/dL | 4% | 2% |
| Elevated glucose levels | |||
| Grade 2 | 6.95–13.88 mmol/L 161–250 mg/dL | 15% | 13% |
| Grade 3 | 13.89–27.75 mmol/L 251 – 500 mg/dL | 4% | 2% |
| Grade 4 | > 27.75 mmol/L > 500 mg/dL | 0% | < 1% |
| HEPATIC PARAMETERS | |||
| Alanine amino transferase | |||
| Grade 2 | 2.6–5 × ULN | 6% | 5% |
| Grade 3 | 5.1–10 × ULN | 3% | 2% |
| Grade 4 | > 10 × ULN | 1% | < 1% |
| Aspartate amino transferase | |||
| Grade 2 | 2.6–5 × ULN | 6% | 8% |
| Grade 3 | 5.1–10 × ULN | 3% | 2% |
| Grade 4 | > 10 × ULN | < 1% | < 1% |
Patients co-infected with hepatitis B and/or hepatitis C virus
In Phase 3 trials TMC125-C206 and TMC125-C216, 139 subjects (12.3%) with chronic hepatitis B and/or hepatitis C virus co-infection out of 1129 subjects were permitted to enroll. AST and ALT abnormalities occurred more frequently in hepatitis B and/or hepatitis C virus co-infected subjects for both treatment groups. Grade 2 or higher laboratory abnormalities that represent a worsening from baseline of AST, ALT or total bilirubin occurred in 27.8%, 25.0% and 7.1% respectively, of INTELENCE®-treated co-infected subjects as compared to 6.7%, 7.5% and 1.8% of non-co-infected INTELENCE®-treated subjects. In general, adverse events reported by INTELENCE®-treated subjects with hepatitis B and/or hepatitis C virus co-infection were similar to INTELENCE®-treated subjects without hepatitis B and/or hepatitis C virus co-infection.
6.2 Clinical Trials Experience: Pediatric Subjects (6 years to less than 18 years of age)
The safety assessment in children and adolescents is based on the Week 24 analysis of the single-arm, Phase 2 trial TMC125-C213 in which 101 antiretroviral treatment-experienced HIV-1 infected subjects 6 years to less than 18 years of age and weighing at least 16 kg received INTELENCE in combination with other antiretroviral agents [see Clinical Studies (14.2)]. The frequency, type and severity of adverse drug reactions in pediatric subjects were comparable to those observed in adult subjects, except for rash which was observed more frequently in pediatric subjects. The most common adverse drug reactions in at least 2% of pediatric subjects were rash and diarrhea. Rash (greater than or equal to Grade 2) occurred in 15% of pediatric subjects. In the majority of cases, rash was mild to moderate, of macular/papular type, and occurred in the second week of therapy. Rash was self-limiting and generally resolved within 1 week on continued therapy. The discontinuation rate for rash was 4%. Rash including serious (Grade 3 or 4) events and discontinuations were more frequently observed in female subjects compared to male subjects.
6.3 Postmarketing Experience
The following events have been identified during postmarketing use of INTELENCE®. Because these events are reported voluntarily from a population of unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune System Disorders: Severe hypersensitivity reactions including cases of hepatic failure have been reported [see Warnings and Precautions (5.1)].
Musculoskeletal and Connective Tissue Disorders: rhabdomyolysis
Skin and Subcutaneous Tissue Disorders: Fatal cases of toxic epidermal necrolysis have been reported [see Warnings and Precautions (5.1)].
7 DRUG INTERACTIONS
Etravirine is a substrate of CYP3A, CYP2C9, and CYP2C19. Therefore, co-administration of INTELENCE® with drugs that induce or inhibit CYP3A, CYP2C9, and CYP2C19 may alter the therapeutic effect or adverse reaction profile of INTELENCE® (see Table 3). [See also Clinical Pharmacology (12.3).]
Etravirine is an inducer of CYP3A and inhibitor of CYP2C9, CYP2C19 and P-glycoprotein. Therefore, co-administration of drugs that are substrates of CYP3A, CYP2C9 and CYP2C19 or are transported by P-glycoprotein with INTELENCE® may alter the therapeutic effect or adverse reaction profile of the co-administered drug(s) (see Table 3). [See also Clinical Pharmacology (12.3).]
Table 3 shows the established and other potentially significant drug interactions based on which, alterations in dose or regimen of INTELENCE® and/or co-administered drug may be recommended. Drugs that are not recommended for co-administration with INTELENCE® are also included in Table 3.
| Concomitant Drug Class: Drug Name | Effect on Concentration of Etravirine or Concomitant Drug | Clinical Comment |
|---|---|---|
| ↑ = increase, ↓ = decrease, ↔ = no change | ||
|
||
| HIV-Antiviral Agents: Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs) | ||
| efavirenz*
nevirapine* | ↓ etravirine | Combining two NNRTIs has not been shown to be beneficial. Concomitant use of INTELENCE® with efavirenz or nevirapine may cause a significant decrease in the plasma concentrations of etravirine and loss of therapeutic effect of INTELENCE®. INTELENCE® and other NNRTIs should not be co-administered. |
| delavirdine | ↑ etravirine | Combining two NNRTIs has not been shown to be beneficial. INTELENCE® and delavirdine should not be co-administered. |
| rilpivirine | ↓ rilpivirine ↔ etravirine | Combining two NNRTIs has not been shown to be beneficial. INTELENCE® and rilpivirine should not be coadministered. |
| HIV-Antiviral Agents: Protease Inhibitors (PIs) | ||
| atazanavir*
(without ritonavir) | ↓ atazanavir | Concomitant use of INTELENCE® with atazanavir without low-dose ritonavir may cause a significant alteration in the plasma concentration of atazanavir. INTELENCE® should not be co-administered with atazanavir without low-dose ritonavir. |
| atazanavir/ritonavir* | ↓ atazanavir ↑ etravirine | Concomitant use of INTELENCE® with atazanavir/ritonavir may cause a significant decrease in atazanavir Cmin and loss of therapeutic effect of atazanavir. In addition, the mean systemic exposure (AUC) of etravirine after co-administration of INTELENCE® with atazanavir/ritonavir is anticipated to be higher than the mean systemic exposure of etravirine observed in the Phase 3 trials after co-administration of INTELENCE® and darunavir/ritonavir (as part of the background regimen). INTELENCE® and atazanavir/ritonavir should not be co-administered. |
| darunavir/ritonavir* | ↓ etravirine | The mean systemic exposure (AUC) of etravirine was reduced when INTELENCE® was co-administered with darunavir/ritonavir. Because all subjects in the Phase 3 trials received darunavir/ritonavir as part of the background regimen and etravirine exposures from these trials were determined to be safe and effective, INTELENCE® and darunavir/ritonavir can be co-administered without dose adjustments. |
| fosamprenavir (without ritonavir) | ↑ amprenavir | Concomitant use of INTELENCE® with fosamprenavir without low-dose ritonavir may cause a significant alteration in the plasma concentration of amprenavir. INTELENCE® should not be co-administered with fosamprenavir without low-dose ritonavir. |
| fosamprenavir/ritonavir* | ↑ amprenavir | Due to a significant increase in the systemic exposure of amprenavir, the appropriate doses of the combination of INTELENCE® and fosamprenavir/ritonavir have not been established. INTELENCE® and fosamprenavir/ritonavir should not be co-administered. |
| indinavir*
(without ritonavir) | ↓ indinavir | Concomitant use of INTELENCE® with indinavir without low-dose ritonavir may cause a significant alteration in the plasma concentration of indinavir. INTELENCE® should not be co-administered with indinavir without low-dose ritonavir. |
| lopinavir/ritonavir* | ↓ etravirine | The mean systemic exposure (AUC) of etravirine was reduced after co-administration of INTELENCE® with lopinavir/ritonavir (tablet). Because the reduction in the mean systemic exposures of etravirine in the presence of lopinavir/ritonavir is similar to the reduction in mean systemic exposures of etravirine in the presence of darunavir/ritonavir, INTELENCE® and lopinavir/ritonavir can be co-administered without dose adjustments. |
| nelfinavir (without ritonavir) | ↑ nelfinavir | Concomitant use of INTELENCE® with nelfinavir without low-dose ritonavir may cause a significant alteration in the plasma concentration of nelfinavir. INTELENCE® should not be co-administered with nelfinavir without low-dose ritonavir. |
| ritonavir* | ↓ etravirine | Concomitant use of INTELENCE® with ritonavir 600 mg twice daily may cause a significant decrease in the plasma concentration of etravirine and loss of therapeutic effect of INTELENCE®. INTELENCE® and ritonavir 600 mg twice daily should not be co-administered. |
| saquinavir/ritonavir* | ↓ etravirine | The mean systemic exposure (AUC) of etravirine was reduced when INTELENCE® was co-administered with saquinavir/ritonavir. Because the reduction in the mean systemic exposures of etravirine in the presence of saquinavir/ritonavir is similar to the reduction in mean systemic exposures of etravirine in the presence of darunavir/ritonavir, INTELENCE® and saquinavir/ritonavir can be co-administered without dose adjustments. |
| tipranavir/ritonavir* | ↓ etravirine | Concomitant use of INTELENCE® with tipranavir/ritonavir may cause a significant decrease in the plasma concentrations of etravirine and loss of therapeutic effect of INTELENCE®. INTELENCE® and tipranavir/ritonavir should not be co-administered. |
| CCR5 Antagonists | ||
| maraviroc* | ↔ etravirine ↓ maraviroc | When INTELENCE® is co-administered with maraviroc in the absence of a potent CYP3A inhibitor (e.g., ritonavir boosted protease inhibitor), the recommended dose of maraviroc is 600 mg twice daily. No dose adjustment of INTELENCE® is needed. |
| maraviroc/darunavir/ritonavir* | ↔ etravirine ↑ maraviroc | When INTELENCE® is co-administered with maraviroc in the presence of a potent CYP3A inhibitor (e.g., ritonavir boosted protease inhibitor), the recommended dose of maraviroc is 150 mg twice daily. No dose adjustment of INTELENCE® is needed. |
| Other Agents | ||
| Antiarrhythmics: digoxin* | ↔ etravirine ↑ digoxin | For patients who are initiating a combination of INTELENCE® and digoxin, the lowest dose of digoxin should initially be prescribed. For patients on a stable digoxin regimen and initiating INTELENCE®, no dose adjustment of either INTELENCE® or digoxin is needed. The serum digoxin concentrations should be monitored and used for titration of the digoxin dose to obtain the desired clinical effect. |
| amiodarone, bepridil, disopyramide, flecainide, lidocaine (systemic), mexiletine, propafenone, quinidine | ↓ antiarrhythmics | Concentrations of these antiarrhythmics may be decreased when co-administered with INTELENCE®. INTELENCE® and antiarrhythmics should be co-administered with caution. Drug concentration monitoring is recommended, if available. |
| Anticoagulants: warfarin | ↑ anticoagulants | Warfarin concentrations may be increased when co-administered with INTELENCE®. The international normalized ratio (INR) should be monitored when warfarin is combined with INTELENCE®. |
| Anticonvulsants: carbamazepine, phenobarbital, phenytoin | ↓ etravirine | Carbamazepine, phenobarbital and phenytoin are inducers of CYP450 enzymes. INTELENCE® should not be used in combination with carbamazepine, phenobarbital, or phenytoin as co-administration may cause significant decreases in etravirine plasma concentrations and loss of therapeutic effect of INTELENCE®. |
| Antifungals: fluconazole*, voriconazole* | ↑ etravirine ↔ fluconazole ↑ voriconazole | Co-administration of etravirine and fluconazole significantly increased etravirine exposures. The amount of safety data at these increased etravirine exposures is limited, therefore, etravirine and fluconazole should be co-administered with caution. No dose adjustment of INTELENCE® or fluconazole is needed. |
| Co-administration of etravirine and voriconazole significantly increased etravirine exposures. The amount of safety data at these increased etravirine exposures is limited, therefore, etravirine and voriconazole should be co-administered with caution. No dose adjustment of INTELENCE® or voriconazole is needed. | ||
| Antifungals: itraconazole, ketoconazole, posaconazole | ↑ etravirine ↓ itraconazole ↓ ketoconazole ↔ posaconazole | Posaconazole, a potent inhibitor of CYP3A4, may increase plasma concentrations of etravirine. Itraconazole and ketoconazole are potent inhibitors as well as substrates of CYP3A4. Concomitant systemic use of itraconazole or ketoconazole and INTELENCE® may increase plasma concentrations of etravirine. Simultaneously, plasma concentrations of itraconazole or ketoconazole may be decreased by INTELENCE®. Dose adjustments for itraconazole, ketoconazole or posaconazole may be necessary depending on the other co-administered drugs. |
| Antiinfectives: clarithromycin* | ↑ etravirine ↓ clarithromycin ↑ 14-OH-clarithromycin | Clarithromycin exposure was decreased by INTELENCE®; however, concentrations of the active metabolite, 14-hydroxy-clarithromycin, were increased. Because 14-hydroxy-clarithromycin has reduced activity against Mycobacterium avium complex (MAC), overall activity against this pathogen may be altered. Alternatives to clarithromycin, such as azithromycin, should be considered for the treatment of MAC. |
| Antimycobacterials:
rifampin, rifapentine | ↓ etravirine | Rifampin and rifapentine are potent inducers of CYP450 enzymes. INTELENCE® should not be used with rifampin or rifapentine as co-administration may cause significant decreases in etravirine plasma concentrations and loss of therapeutic effect of INTELENCE®. |
| Antimycobacterials:
rifabutin* | ↓ etravirine ↓ rifabutin ↓ 25-O-desacetylrifabutin | If INTELENCE® is NOT co-administered with a protease inhibitor/ritonavir, then rifabutin at a dose of 300 mg once daily is recommended. If INTELENCE® is co-administered with darunavir/ritonavir, lopinavir/ritonavir or saquinavir/ritonavir, then rifabutin should not be co-administered due to the potential for significant reductions in etravirine exposure. |
| Benzodiazepines:
diazepam | ↑ diazepam | Concomitant use of INTELENCE® with diazepam may increase plasma concentrations of diazepam. A decrease in diazepam dose may be needed. |
| Corticosteroids: dexamethasone (systemic) | ↓ etravirine | Systemic dexamethasone induces CYP3A and can decrease etravirine plasma concentrations. This may result in loss of therapeutic effect of INTELENCE®. Systemic dexamethasone should be used with caution or alternatives should be considered, particularly for long-term use. |
| Herbal Products: St. John's wort (Hypericum perforatum) | ↓ etravirine | Concomitant use of INTELENCE® with products containing St. John's wort may cause significant decreases in etravirine plasma concentrations and loss of therapeutic effect of INTELENCE®. INTELENCE® and products containing St. John's wort should not be co-administered. |
| HMG-CoA
Reductase Inhibitors: atorvastatin* fluvastatin, lovastatin, pitavastatin, pravastatin, rosuvastatin, simvastatin | ↔ etravirine ↓ atorvastatin ↑ 2-OH-atorvastatin ↔ etravirine ↑ fluvastatin, ↓ lovastatin, ↑ pitavastatin, ↔ pravastatin, ↔ rosuvastatin, ↓ simvastatin | The combination of INTELENCE® and atorvastatin can be given without dose adjustments, however, the dose of atorvastatin may need to be altered based on clinical response. No interaction between pravastatin, rosuvastatin and INTELENCE® is expected. Lovastatin and simvastatin are CYP3A substrates and co-administration with INTELENCE® may result in lower plasma concentrations of the HMG-CoA reductase inhibitor. Fluvastatin and pitavastatin are metabolized by CYP2C9 and co-administration with INTELENCE® may result in higher plasma concentrations of the HMG-CoA reductase inhibitor. Dose adjustments for these HMG-CoA reductase inhibitors may be necessary. |
| Immunosuppressants: cyclosporine, sirolimus, tacrolimus | ↓ immunosuppressant | INTELENCE® and systemic immunosuppressants should be co-administered with caution because plasma concentrations of cyclosporine, sirolimus, or tacrolimus may be affected. |
| Narcotic Analgesics/Treatment of Opioid Dependence: buprenorphine, buprenorphine/naloxone*, methadone* | ↔ etravirine ↓ buprenorphine ↔ norbuprenorphine ↔ methadone | INTELENCE® and buprenorphine (or buprenorphine/naloxone) can be co-administered without dose adjustments, however, clinical monitoring for withdrawal symptoms is recommended as buprenorphine (or buprenorphine/naloxone) maintenance therapy may need to be adjusted in some patients. INTELENCE® and methadone can be co-administered without dose adjustments, however, clinical monitoring for withdrawal symptoms is recommended as methadone maintenance therapy may need to be adjusted in some patients. |
| Phosphodiesterase Type 5
(PDE-5) Inhibitors: sildenafil*, tadalafil, vardenafil | ↓ sildenafil ↓ N-desmethyl-sildenafil | INTELENCE® and sildenafil can be co-administered without dose adjustments, however, the dose of sildenafil may need to be altered based on clinical effect. |
| Platelet Aggregation Inhibitors:
clopidogrel | ↓ clopidogrel (active) metabolite | Activation of clopidogrel to its active metabolite may be decreased when clopidogrel is co-administered with INTELENCE®. Alternatives to clopidogrel should be considered. |
In addition to the drugs included in Table 3, the interaction between INTELENCE® and the following drugs were evaluated in clinical studies and no dose adjustment is needed for either drug [see Clinical Pharmacology (12.3)]: didanosine, enfuvirtide (ENF), ethinylestradiol/norethindrone, omeprazole, paroxetine, raltegravir, ranitidine, and tenofovir disoproxil fumarate.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B
No adequate and well-controlled studies of INTELENCE® use in pregnant women have been conducted. In addition, no pharmacokinetic studies have been conducted in pregnant patients. INTELENCE® should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Antiretroviral Pregnancy Registry
To monitor maternal-fetal outcomes of pregnant women exposed to INTELENCE®, an Antiretroviral Pregnancy Registry has been established. Physicians are encouraged to register patients by calling 1-800-258-4263.
Animal Data
Reproductive and developmental toxicity studies were performed in rabbits (at oral doses up to 375 mg per kg per day) and rats (at oral doses up to 1000 mg per kg per day). In both species, no treatment-related embryo-fetal effects including malformations were observed. In addition, no treatment-related effects were observed in a separate pre- and postnatal study performed in rats at oral doses up to 500 mg per kg per day. The systemic drug exposures achieved in these animal studies were equivalent to those at the recommended human dose (400 mg per day).
8.3 Nursing mothers
The Centers for Disease Control and Prevention recommend that HIV-infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV. It is not known whether etravirine is secreted in human milk. Because of both the potential for HIV transmission and the potential for adverse reactions in nursing infants, mothers should be instructed not to breastfeed if they are receiving INTELENCE®.
8.4 Pediatric use
Treatment with INTELENCE® is not recommended in children less than 6 years of age. The pharmacokinetics, safety, tolerability and efficacy of INTELENCE® in children less than 6 years of age have not been established [see Clinical Pharmacology (12.3)].
The safety, pharmacokinetic profile, and virologic and immunologic responses of INTELENCE® were evaluated in treatment-experienced HIV-1-infected pediatric subjects 6 years to less than 18 years of age and weighing at least 16 kg [see Adverse Reactions (6.2), Clinical Pharmacology (12.3) and Clinical Studies (14.2)]. Frequency, type, and severity of adverse drug reactions in pediatric subjects were comparable to those observed in adults, except for rash [see Adverse Reactions (6.2)]. Please see Dosage and Administration (2.2) for dosing recommendations for pediatric subjects 6 years to less than 18 years of age and weighing at least 16 kg.
8.5 Geriatric use
Clinical studies of INTELENCE® did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger subjects. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Hepatic Impairment
No dose adjustment of INTELENCE® is required in patients with mild (Child-Pugh Class A) or moderate (Child-Pugh Class B) hepatic impairment. The pharmacokinetics of INTELENCE® have not been evaluated in patients with severe hepatic impairment (Child-Pugh Class C).
8.7 Renal Impairment
Since the renal clearance of etravirine is negligible (less than 1.2%), a decrease in total body clearance is not expected in patients with renal impairment. No dose adjustments are required in patients with renal impairment. As etravirine is highly bound to plasma proteins, it is unlikely that it will be significantly removed by hemodialysis or peritoneal dialysis.
10 OVERDOSAGE
There is no specific antidote for overdose with INTELENCE®. Human experience of overdose with INTELENCE® is limited. The highest dose studied in healthy volunteers was 400 mg once daily. Treatment of overdose with INTELENCE® consists of general supportive measures including monitoring of vital signs and observation of the clinical status of the patient. If indicated, elimination of unabsorbed active substance is to be achieved by emesis or gastric lavage. Administration of activated charcoal may also be used to aid in removal of unabsorbed active substance. Because etravirine is highly protein bound, dialysis is unlikely to result in significant removal of the active substance.
11 DESCRIPTION
INTELENCE® (etravirine) is a non-nucleoside reverse transcriptase inhibitor (NNRTI) of human immunodeficiency virus type 1 (HIV-1).
The chemical name for etravirine is 4-[[6-amino-5-bromo-2-[(4-cyanophenyl)amino]-4-pyrimidinyl]oxy]-3,5-dimethylbenzonitrile. Its molecular formula is C20H15BrN6O and its molecular weight is 435.28. Etravirine has the following structural formula:
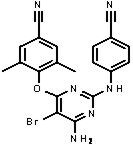
Etravirine is a white to slightly yellowish brown powder. Etravirine is practically insoluble in water over a wide pH range. It is very slightly soluble in propylene glycol and slightly soluble in ethanol. Etravirine is soluble in polyethylene glycol (PEG)400 and freely soluble in some organic solvents (e.g., N,N-dimethylformamide and tetrahydrofuran).
INTELENCE® 25 mg tablets are available as white to off-white, oval scored tablets for oral administration. Each 25 mg tablet contains 25 mg of etravirine and the inactive ingredients hypromellose, microcrystalline cellulose, colloidal silicon dioxide, croscarmellose sodium, magnesium stearate and lactose monohydrate.
INTELENCE® 100 mg tablets are available as white to off-white, oval tablets for oral administration. Each 100 mg tablet contains 100 mg of etravirine and the inactive ingredients hypromellose, microcrystalline cellulose, colloidal silicon dioxide, croscarmellose sodium, magnesium stearate and lactose monohydrate.
INTELENCE® 200 mg tablets are available as white to off-white, biconvex, oblong tablets for oral administration. Each 200 mg tablet contains 200 mg of etravirine and the inactive ingredients hypromellose, silicified microcrystalline cellulose, microcrystalline cellulose, colloidal silicon dioxide, croscarmellose sodium and magnesium stearate.
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
Effects on Electrocardiogram
In a randomized, double-blind, active, and placebo-controlled crossover study, 41 healthy subjects were administered INTELENCE® 200 mg twice daily, INTELENCE® 400 mg once daily, placebo, and moxifloxacin 400 mg. After 8 days of dosing, etravirine did not prolong the QT interval. The maximum mean (upper 1-sided 95% CI) baseline and placebo-adjusted QTcF were 0.6 ms (3.3 ms) for 200 mg twice daily and -1.0 ms (2.5 ms) for 400 mg once daily dosing regimens.
12.3 Pharmacokinetics
Pharmacokinetics in Adults
The pharmacokinetic properties of INTELENCE® were determined in healthy adult subjects and in treatment-experienced HIV-1-infected adult and pediatric subjects. The systemic exposures (AUC) to etravirine were lower in HIV-1-infected subjects than in healthy subjects.
| Parameter | Etravirine 200 mg twice daily N = 575 |
|---|---|
|
|
| AUC12h (ng∙h/mL) | |
| Geometric Mean ± Standard Deviation | 4522 ± 4710 |
| Median (Range) | 4380 (458 – 59084) |
| C0h (ng/mL) | |
| Geometric Mean ± Standard Deviation | 297 ± 391 |
| Median (Range) | 298 (2 – 4852) |
Note: The median protein binding adjusted EC50 for MT4 cells infected with HIV-1/IIIB in vitro equals 4 ng per mL.
Absorption and Bioavailability
Following oral administration, etravirine was absorbed with a Tmax of about 2.5 to 4 hours. The absolute oral bioavailability of INTELENCE® is unknown.
In healthy subjects, the absorption of etravirine is not affected by co-administration of oral ranitidine or omeprazole, drugs that increase gastric pH.
Effects of Food on Oral Absorption
The systemic exposure (AUC) to etravirine was decreased by about 50% when INTELENCE® was administered under fasting conditions, as compared to when INTELENCE® was administered following a meal. Therefore, INTELENCE® should always be taken following a meal. Within the range of meals studied, the systemic exposures to etravirine were similar. The total caloric content of the various meals evaluated ranged from 345 kilocalories (17 grams fat) to 1160 kilocalories (70 grams fat) [see Dosage and Administration (2)].
Distribution
Etravirine is about 99.9% bound to plasma proteins, primarily to albumin (99.6%) and alpha 1-acid glycoprotein (97.66% to 99.02%) in vitro. The distribution of etravirine into compartments other than plasma (e.g., cerebrospinal fluid, genital tract secretions) has not been evaluated in humans.
Metabolism
In vitro experiments with human liver microsomes (HLMs) indicate that etravirine primarily undergoes metabolism by CYP3A, CYP2C9, and CYP2C19 enzymes. The major metabolites, formed by methyl hydroxylation of the dimethylbenzonitrile moiety, were at least 90% less active than etravirine against wild-type HIV in cell culture.
Elimination
After single dose oral administration of 800 mg 14C-etravirine, 93.7% and 1.2% of the administered dose of 14C-etravirine was recovered in the feces and urine, respectively. Unchanged etravirine accounted for 81.2% to 86.4% of the administered dose in feces. Unchanged etravirine was not detected in urine. The mean (± standard deviation) terminal elimination half-life of etravirine was about 41 (± 20) hours.
Special Populations
Hepatic Impairment
Etravirine is primarily metabolized by the liver. The steady state pharmacokinetic parameters of etravirine were similar after multiple dose administration of INTELENCE® to subjects with normal hepatic function (16 subjects), mild hepatic impairment (Child-Pugh Class A, 8 subjects), and moderate hepatic impairment (Child-Pugh Class B, 8 subjects). The effect of severe hepatic impairment on the pharmacokinetics of etravirine has not been evaluated.
Hepatitis B and/or Hepatitis C Virus Co-infection
Population pharmacokinetic analysis of the TMC125-C206 and TMC125-C216 trials showed reduced clearance for etravirine in HIV-1-infected subjects with hepatitis B and/or C virus co-infection. Based upon the safety profile of INTELENCE® [see Adverse Reactions (6)], no dose adjustment is necessary in patients co-infected with hepatitis B and/or C virus.
Renal Impairment
The pharmacokinetics of etravirine have not been studied in patients with renal impairment. The results from a mass balance study with 14C-etravirine showed that less than 1.2% of the administered dose of etravirine is excreted in the urine as metabolites. No unchanged drug was detected in the urine. As etravirine is highly bound to plasma proteins, it is unlikely that it will be significantly removed by hemodialysis or peritoneal dialysis.
Race
Population pharmacokinetic analysis of etravirine in HIV-infected subjects did not show an effect of race on exposure to etravirine.
Geriatric Patients
Population pharmacokinetic analysis in HIV-infected subjects showed that etravirine pharmacokinetics are not considerably different within the age range (18 to 77 years) evaluated [see Use in Specific Populations (8.5)].
Pediatric Patients
The pharmacokinetics of etravirine in 101 treatment-experienced HIV-1-infected pediatric subjects, 6 years to less than 18 years of age and weighing at least 16 kg showed that the administered weight-based dosages (approximately 5.2 mg per kg twice daily up to the adult recommended doses) resulted in etravirine exposure comparable to that in adults receiving INTELENCE® 200 mg twice daily [see Dosage and Administration (2.2)] when administered at a dose corresponding to 5.2 mg per kg twice daily. The population pharmacokinetic estimates for etravirine AUC12h and C0h are summarized in the table below.
| Parameter | N = 101 |
|---|---|
| AUC12h (ng•h/ml) | |
| Geometric Mean ± Standard Deviation | 3742 ± 4314 |
| Median (Range) | 4499 (62 – 28865) |
| C0h (ng•h/ml) | |
| Geometric Mean ± Standard Deviation | 205 ± 342 |
| Median (Range) | 287 (2 – 2276) |
The pharmacokinetics of etravirine in pediatric subjects less than 6 years of age have not been established.
Drug Interactions
[See also Drug Interactions (7).]
Etravirine is a substrate of CYP3A, CYP2C9, and CYP2C19. Therefore, co-administration of INTELENCE® with drugs that induce or inhibit CYP3A, CYP2C9, and CYP2C19 may alter the therapeutic effect or adverse reaction profile of INTELENCE®.
Etravirine is an inducer of CYP3A and inhibitor of CYP2C9, CYP2C19 and P-glycoprotein. Therefore, co-administration of drugs that are substrates of CYP3A, CYP2C9 and CYP2C19 or are transported by P-glycoprotein with INTELENCE® may alter the therapeutic effect or adverse reaction profile of the co-administered drug(s).
Drug interaction studies were performed with INTELENCE® and other drugs likely to be co-administered and some drugs commonly used as probes for pharmacokinetic interactions. The effects of co-administration of other drugs on the AUC, Cmax, and Cmin values of etravirine are summarized in Table 5 (effect of other drugs on INTELENCE®). The effect of co-administration of INTELENCE® on the AUC, Cmax, and Cmin values of other drugs are summarized in Table 6 (effect of INTELENCE® on other drugs). For information regarding clinical recommendations, see Drug Interactions (7).
| Co-administered Drug | Dose/Schedule of Co-administered Drug | N | Exposure | Mean Ratio of Etravirine
Pharmacokinetic Parameters 90% CI; No Effect = 1.00 |
||
|---|---|---|---|---|---|---|
| Cmax | AUC | Cmin | ||||
| CI = Confidence Interval; N = number of subjects with data; N.A. = not available; ↑ = increase; ↓ = decrease; ↔ = no change; q.d. = once daily; b.i.d. = twice daily; q.a.m. = once daily in the morning | ||||||
|
||||||
| Co-Administration With Protease Inhibitors (PIs) | ||||||
| Atazanavir | 400 mg q.d. | 14 | ↑ | 1.47 (1.36–1.59) | 1.50 (1.41–1.59) | 1.58 (1.46–1.70) |
| Atazanavir/ ritonavir | 300/100 mg q.d. | 14 | ↑ | 1.30 (1.17–1.44) | 1.30 (1.18–1.44) | 1.26 (1.12–1.42) |
| Darunavir/ ritonavir | 600/100 mg b.i.d. | 14 | ↓ | 0.68 (0.57–0.82) | 0.63 (0.54–0.73) | 0.51 (0.44–0.61) |
| Lopinavir/ ritonavir (tablet) | 400/100 mg b.i.d. | 16 | ↓ | 0.70 (0.64–0.78) | 0.65 (0.59–0.71) | 0.55 (0.49–0.62) |
| Ritonavir | 600 mg b.i.d. | 11 | ↓ | 0.68 (0.55–0.85) | 0.54 (0.41–0.73) | N.A. |
| Saquinavir/ ritonavir | 1000/100 mg b.i.d. | 14 | ↓ | 0.63 (0.53–0.75) | 0.67 (0.56–0.80) | 0.71 (0.58–0.87) |
| Tipranavir/ ritonavir | 500/200 mg b.i.d. | 19 | ↓ | 0.29 (0.22–0.40) | 0.24 (0.18–0.33) | 0.18 (0.13–0.25) |
| Co-Administration With Nucleoside Reverse Transcriptase Inhibitors (NRTIs) | ||||||
| Didanosine | 400 mg q.d. | 15 | ↔ | 1.16 (1.02–1.32) | 1.11 (0.99–1.25) | 1.05 (0.93–1.18) |
| Tenofovir disoproxil fumarate | 300 mg q.d. | 23 | ↓ | 0.81 (0.75–0.88) | 0.81 (0.75–0.88) | 0.82 (0.73–0.91) |
| Co-Administration With CCR5 Antagonists | ||||||
| Maraviroc | 300 mg b.i.d. | 14 | ↔ | 1.05 (0.95–1.17) | 1.06 (0.99–1.14) | 1.08 (0.98–1.19) |
| Maraviroc (when co-administered with darunavir/ritonavir)* | 150/600/100 mg b.i.d. | 10 | ↔ | 1.08 (0.98–1.20) | 1.00 (0.86–1.15) | 0.81 (0.65–1.01) |
| Co-Administration With Integrase Strand Transfer Inhibitors | ||||||
| Raltegravir | 400 mg b.i.d. | 19 | ↔ | 1.04 (0.97–1.12) | 1.10 (1.03–1.16) | 1.17 (1.10–1.26) |
| Co-Administration With Other Drugs | ||||||
| Atorvastatin | 40 mg q.d. | 16 | ↔ | 0.97 (0.93–1.02) | 1.02 (0.97–1.07) | 1.10 (1.02–1.19) |
| Clarithromycin | 500 mg b.i.d. | 15 | ↑ | 1.46 (1.38–1.56) | 1.42 (1.34–1.50) | 1.46 (1.36–1.58) |
| Fluconazole | 200 mg q.a.m. | 16 | ↑ | 1.75 (1.60–1.91) | 1.86 (1.73–2.00) | 2.09 (1.90–2.31) |
| Omeprazole | 40 mg q.d. | 18 | ↑ | 1.17 (0.96–1.43) | 1.41 (1.22–1.62) | N.A. |
| Paroxetine | 20 mg q.d. | 16 | ↔ | 1.05 (0.96–1.15) | 1.01 (0.93–1.10) | 1.07 (0.98–1.17) |
| Ranitidine | 150 mg b.i.d. | 18 | ↓ | 0.94 (0.75–1.17) | 0.86 (0.76–0.97) | N.A. |
| Rifabutin | 300 mg q.d. | 12 | ↓ | 0.63 (0.53–0.74) | 0.63 (0.54–0.74) | 0.65 (0.56–0.74) |
| Voriconazole | 200 mg b.i.d. | 16 | ↑ | 1.26 (1.16–1.38) | 1.36 (1.25–1.47) | 1.52 (1.41–1.64) |
| Co-administered Drug | Dose/Schedule of Co-administered Drug | N | Exposure | Mean Ratio of Co-administered Drug Pharmacokinetic Parameters 90% CI; No effect = 1.00 |
||
|---|---|---|---|---|---|---|
| Cmax | AUC | Cmin | ||||
| CI = Confidence Interval; N = number of subjects with data; N.A. = not available; ↑ = increase; ↓ = decrease; ↔ = no change; q.d. = once daily ; b.i.d. = twice daily; q.a.m. = once daily in the morning | ||||||
|
||||||
| Co-Administration With Protease Inhibitors (PIs) | ||||||
| Atazanavir | 400 mg q.d. | 14 | ↓ | 0.97 (0.73–1.29) | 0.83 (0.63–1.09) | 0.53 (0.38–0.73) |
| Atazanavir/ ritonavir | 300/100 mg q.d. | 13 | ↓ | 0.97 (0.89–1.05) | 0.86 (0.79–0.93) | 0.62 (0.55–0.71) |
| Darunavir/ ritonavir | 600/100 mg b.i.d. | 15 | ↔ | 1.11 (1.01–1.22) | 1.15 (1.05–1.26) | 1.02 (0.90–1.17) |
| Fosamprenavir/ ritonavir | 700/100 mg b.i.d. | 8 | ↑ | 1.62 (1.47–1.79) | 1.69 (1.53–1.86) | 1.77 (1.39–2.25) |
| Lopinavir/ ritonavir (tablet) | 400/100 mg b.i.d. | 16 | ↔ | 0.89 (0.82–0.96) | 0.87 (0.83–0.92) | 0.80 (0.73–0.88) |
| Saquinavir/ ritonavir | 1000/100 mg b.i.d. | 15 | ↔ | 1.00 (0.70–1.42) | 0.95 (0.64–1.42) | 0.80 (0.46–1.38) |
| Tipranavir/ ritonavir | 500/200 mg b.i.d. | 19 | ↑ | 1.14 (1.02–1.27) | 1.18 (1.03–1.36) | 1.24 (0.96–1.59) |
| Co-Administration With Nucleoside Reverse Transcriptase Inhibitors (NRTIs) | ||||||
| Didanosine | 400 mg q.d. | 14 | ↔ | 0.91 (0.58–1.42) | 0.99 (0.79–1.25) | N.A. |
| Tenofovir disoproxil fumarate | 300 mg q.d. | 19 | ↔ | 1.15 (1.04–1.27) | 1.15 (1.09–1.21) | 1.19 (1.13–1.26) |
| Co-Administration With CCR5 Antagonists | ||||||
| Maraviroc | 300 mg b.i.d. | 14 | ↓ | 0.40 (0.28–0.57) | 0.47 (0.38–0.58) | 0.61 (0.53–0.71) |
| Maraviroc (when co-administered with darunavir/ritonavir)* | 150/600/100 mg b.i.d. | 10 | ↑ | 1.77 (1.20–2.60) | 3.10 (2.57–3.74) | 5.27 (4.51–6.15) |
| Co-Administration With Integrase Strand Transfer Inhibitors | ||||||
| Raltegravir | 400 mg b.i.d. | 19 | ↓ | 0.89 (0.68–1.15) | 0.90 (0.68–1.18) | 0.66 (0.34–1.26) |
| Co-Administration With Other Drugs | ||||||
| Atorvastatin | 40 mg q.d. | 16 | ↓ | 1.04 (0.84–1.30) | 0.63 (0.58–0.68) | N.A. |
| 2-hydroxy-atorvastatin | 16 | ↑ | 1.76 (1.60–1.94) | 1.27 (1.19–1.36) | N.A. | |
| Buprenorphine | Individual dose regimen ranging from 4/1 mg to 16/4 mg q.d. | 16 | ↓ | 0.89 (0.76–1.05) | 0.75 (0.66–0.84) | 0.60 (0.52–0.68) |
| Norbuprenorphine | 16 | ↔ | 1.08 (0.95–1.23) | 0.88 (0.81–0.96) | 0.76 (0.67–0.87) |
|
| Clarithromycin | 500 mg b.i.d. | 15 | ↓ | 0.66 (0.57–0.77) | 0.61 (0.53–0.69) | 0.47 (0.38–0.57) |
| 14-hydroxy-clarithromycin | 15 | ↑ | 1.33 (1.13–1.56) | 1.21 (1.05–1.39) | 1.05 (0.90–1.22) |
|
| Digoxin | 0.5 mg single dose | 16 | ↑ | 1.19 (0.96–1.49) | 1.18 (0.90–1.56) | N.A. |
| Ethinylestradiol | 0.035 mg q.d. | 16 | ↑ | 1.33 (1.21–1.46) | 1.22 (1.13–1.31) | 1.09 (1.01–1.18) |
| Norethindrone | 1 mg q.d. | 16 | ↔ | 1.05 (0.98–1.12) | 0.95 (0.90–0.99) | 0.78 (0.68–0.90) |
| Fluconazole | 200 mg q.a.m. | 15 | ↔ | 0.92 (0.85–1.00) | 0.94 (0.88–1.01) | 0.91 (0.84–0.98) |
| R(-) Methadone | Individual dose regimen ranging from 60 to 130 mg/day | 16 | ↔ | 1.02 (0.96–1.09) | 1.06 (0.99–1.13) | 1.10 (1.02–1.19) |
| S(+) Methadone | 16 | ↔ | 0.89 (0.83–0.97) | 0.89 (0.82–0.96) | 0.89 (0.81–0.98) |
|
| Paroxetine | 20 mg q.d. | 16 | ↔ | 1.06 (0.95–1.20) | 1.03 (0.90–1.18) | 0.87 (0.75–1.02) |
| Rifabutin | 300 mg q.d. | 12 | ↓ | 0.90 (0.78–1.03) | 0.83 (0.75–0.94) | 0.76 (0.66–0.87) |
| 25-O-desacetylrifabutin | 300 mg q.d. | 12 | ↓ | 0.85 (0.72–1.00) | 0.83 (0.74–0.92) | 0.78 (0.70–0.87) |
| Sildenafil | 50 mg single dose | 15 | ↓ | 0.55 (0.40–0.75) | 0.43 (0.36–0.51) | N.A. |
| N-desmethyl-sildenafil | 15 | ↓ | 0.75 (0.59–0.96) | 0.59 (0.52–0.68) | N.A. | |
| Voriconazole | 200 mg b.i.d. | 14 | ↑ | 0.95 (0.75–1.21) | 1.14 (0.88–1.47) | 1.23 (0.87–1.75) |
12.4 Microbiology
Mechanism of Action
Etravirine is an NNRTI of human immunodeficiency virus type 1 (HIV-1). Etravirine binds directly to reverse transcriptase (RT) and blocks the RNA-dependent and DNA-dependent DNA polymerase activities by causing a disruption of the enzyme's catalytic site. Etravirine does not inhibit the human DNA polymerases α, β, and γ.
Antiviral Activity in Cell Culture
Etravirine exhibited activity against laboratory strains and clinical isolates of wild-type HIV-1 in acutely infected T-cell lines, human peripheral blood mononuclear cells, and human monocytes/macrophages with median EC50 values ranging from 0.9 to 5.5 nM (i.e., 0.4 to 2.4 ng per mL). Etravirine demonstrated antiviral activity in cell culture against a broad panel of HIV-1 group M isolates (subtype A, B, C, D, E, F, G) with EC50 values ranging from 0.29 to 1.65 nM and EC50 values ranging from 11.5 to 21.7 nM against group O primary isolates. Etravirine did not show antagonism when studied in combination with the following antiretroviral drugs—the NNRTIs delavirdine, efavirenz, and nevirapine; the N(t)RTIs abacavir, didanosine, emtricitabine, lamivudine, stavudine, tenofovir, zalcitabine, and zidovudine; the PIs amprenavir, atazanavir, darunavir, indinavir, lopinavir, nelfinavir, ritonavir, saquinavir, and tipranavir; the fusion inhibitor enfuvirtide; the integrase strand transfer inhibitor raltegravir and the CCR5 co-receptor antagonist maraviroc.
Resistance
In Cell Culture
Etravirine-resistant strains were selected in cell culture originating from wild-type HIV-1 of different origins and subtypes, as well as NNRTI resistant HIV-1. Development of reduced susceptibility to etravirine typically required more than one substitution in reverse transcriptase of which the following were observed most frequently: L100I, E138K, E138G, V179I, Y181C, and M230I.
In Treatment-Experienced Subjects
In the Phase 3 trials TMC125-C206 and TMC125-C216, substitutions that developed most commonly in subjects with virologic failure at Week 48 to the INTELENCE®-containing regimen were V179F, V179I, and Y181C which usually emerged in a background of multiple other NNRTI resistance-associated substitutions. In all the trials conducted with INTELENCE® in HIV-1 infected subjects, the following substitutions emerged most commonly: L100I, E138G, V179F, V179I, Y181C and H221Y. Other NNRTI-resistance associated substitutions which emerged on etravirine treatment in less than 10% of the virologic failure isolates included K101E/H/P, K103N/R, V106I/M, V108I, Y181I, Y188L, V189I, G190S/C, N348I and R356K. The emergence of NNRTI substitutions on etravirine treatment contributed to decreased susceptibility to etravirine with a median fold-change in etravirine susceptibility of 40-fold from reference and a median fold-change of 6-fold from baseline.
Cross-Resistance
Site-Directed NNRTI Mutant Virus
Etravirine showed antiviral activity against 55 of 65 HIV-1 strains (85%) with single amino acid substitutions at RT positions associated with NNRTI resistance, including the most commonly found K103N. The single amino acid substitutions associated with an etravirine reduction in susceptibility greater than 3-fold were K101A, K101P, K101Q, E138G, E138Q, Y181C, Y181I, Y181T, Y181V, and M230L, and of these, the greatest reductions were Y181I (13-fold change in EC50 value) and Y181V (17-fold change in EC50 value). Mutant strains containing a single NNRTI resistance associated substitution (K101P, K101Q, E138Q, or M230L) had cross-resistance between etravirine and efavirenz. The majority (39 of 61; 64%) of the NNRTI mutant viruses with 2 or 3 amino acid substitutions associated with NNRTI resistance had decreased susceptibility to etravirine (fold-change greater than 3). The highest levels of resistance to etravirine were observed for HIV-1 harboring a combination of substitutions V179F + Y181C (187 fold-change), V179F + Y181I (123 fold-change), or V179F + Y181C + F227C (888 fold-change).
Clinical Isolates
Etravirine retained a fold-change less than or equal to 3 against 60% of 6171 NNRTI-resistant clinical isolates. In the same panel, the proportion of clinical isolates resistant to delavirdine, efavirenz and/or nevirapine (defined as a fold-change above their respective biological cutoff values in the assay) was 79%, 87%, and 95%, respectively. In TMC125-C206 and TMC125-C216, 34% of the baseline isolates had decreased susceptibility to etravirine (fold-change greater than 3) and 60%, 69%, and 78% of all baseline isolates were resistant to delavirdine, efavirenz, and nevirapine, respectively. Of subjects who received etravirine and were virologic failures in TMC125-C206 and TMC125-C216, 90%, 84%, and 96% of viral isolates obtained at the time of treatment failure were resistant to delavirdine, efavirenz, and nevirapine, respectively. Therefore, cross-resistance to delavirdine, efavirenz, and/or nevirapine is expected after virologic failure with an etravirine-containing regimen for the virologic failure isolates.
Treatment-naïve HIV-1-infected subjects in the Phase 3 trials for EDURANT (rilpivirine)
There are currently no clinical data available on the use of etravirine in subjects who experienced virologic failure on a rilpivirine-containing regimen. However, in the rilpivirine adult clinical development program, there was evidence of phenotypic cross-resistance between rilpivirine and etravirine. In the pooled analyses of the Phase 3 clinical trials for rilpivirine, 38 rilpivirine virologic failure subjects had evidence of HIV-1 strains with genotypic and phenotypic resistance to rilpivirine. Of these subjects, 89% (34 subjects) of virologic failure isolates were cross-resistant to etravirine based on phenotype data. Consequently, it can be inferred that cross-resistance to etravirine is likely after virologic failure and development of rilpivirine resistance. Refer to the prescribing information for EDURANT (rilpivirine) for further information.
Baseline Genotype/Phenotype and Virologic Outcome Analyses
In TMC125-C206 and TMC125-C216, the presence at baseline of the substitutions L100I, E138A, I167V, V179D, V179F, Y181I, Y181V, or G190S was associated with a decreased virologic response to etravirine. Additional substitutions associated with a decreased virologic response to etravirine when in the presence of 3 or more additional 2008 IAS-USA defined NNRTI substitutions include A98G, K101H, K103R, V106I, V179T, and Y181C. The presence of K103N, which was the most prevalent NNRTI substitution in TMC125-C206 and TMC125-C216 at baseline, did not affect the response in the INTELENCE® arm. Overall, response rates to etravirine decreased as the number of baseline NNRTI substitutions increased (shown as the proportion of subjects achieving viral load less than 50 plasma HIV RNA copies per mL at Week 48) (Table 7).
| # IAS-USA-Defined NNRTI substitutions* | Etravirine Arms N = 561 |
|
|---|---|---|
| Re-Used/Not Used Enfuvirtide | De Novo Enfuvirtide | |
|
||
| All ranges | 61% (254/418) | 76% (109/143) |
| 0 | 68% (52/76) | 95% (20/21) |
| 1 | 67% (72/107) | 77% (24/31) |
| 2 | 64% (75/118) | 86% (38/44) |
| 3 | 55% (36/65) | 62% (16/26) |
| ≥ 4 | 37% (19/52) | 52% (11/21) |
| Placebo Arms
N = 592 |
||
| All ranges | 34% (147/435) | 59% (93/157) |
Response rates assessed by baseline etravirine phenotype are shown in Table 8. These baseline phenotype groups are based on the select subject populations in TMC125-C206 and TMC125-C216 and are not meant to represent definitive clinical susceptibility breakpoints for INTELENCE®. The data are provided to give clinicians information on the likelihood of virologic success based on pre-treatment susceptibility to etravirine in treatment-experienced patients.
| Etravirine Fold Change | Etravirine Arms N = 559 |
||
|---|---|---|---|
| Re-Used/Not Used Enfuvirtide | De Novo Enfuvirtide | Clinical Response Range | |
|
|||
| All ranges | 61% (253/416) | 76% (109/143) | Overall Response |
| 0 – 3 | 69% (188/274) | 83% (75/90) | Higher than Overall Response |
| > 3 – 13 | 50% (39/78) | 66% (25/38) | Lower than Overall Response |
| > 13 | 41% (26/64) | 60% (9/15) | Lower than Overall Response |
| Placebo Arms
N = 583 |
|||
| All ranges | 34% (145/429) | 60% (92/154) | |
The proportion of virologic responders (viral load less than 50 HIV-1 RNA copies per mL) by the phenotypic susceptibility score (PSS) of the background therapy, including enfuvirtide, is shown in Table 9.
| INTELENCE® + BR N=559 | Placebo + BR N=586 |
|
|---|---|---|
|
||
| PSS* | ||
| 0 | 43% (40/93) | 5% (5/95) |
| 1 | 61% (125/206) | 28% (64/226) |
| 2 | 77% (114/149) | 59% (97/165) |
| ≥ 3 | 75% (83/111) | 72% (72/100) |
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Etravirine was evaluated for carcinogenic potential by oral gavage administration to mice and rats for up to approximately 104 weeks. Daily doses of 50, 200 and 400 mg per kg were administered to mice and doses of 70, 200 and 600 mg per kg were administered to rats in the initial period of approximately 41 to 52 weeks. The high and middle doses were subsequently adjusted due to tolerability and reduced by 50% in mice and by 50 to 66% in rats to allow for completion of the studies. In the mouse study, statistically significant increases in the incidences of hepatocellular carcinoma and incidences of hepatocellular adenomas or carcinomas combined were observed in treated females. In the rat study, no statistically significant increases in tumor findings were observed in either sex. The relevance of these liver tumor findings in mice to humans is not known. Because of tolerability of the formulation in these rodent studies, maximum systemic drug exposures achieved at the doses tested were lower than those in humans at the clinical dose (400 mg per day), with animal vs. human AUC ratios being 0.6-fold (mice) and 0.2–0.7-fold (rats).
Mutagenesis
Etravirine tested negative in the in vitro Ames reverse mutation assay, in vitro chromosomal aberration assay in human lymphocyte, and in vitro clastogenicity mouse lymphoma assay, tested in the absence and presence of a metabolic activation system. Etravirine did not induce chromosomal damage in the in vivo micronucleus test in mice.
14 CLINICAL STUDIES
14.1 Treatment-Experienced Adult Subjects
The clinical efficacy of INTELENCE® is derived from the analyses of 48-week data from 2 ongoing, randomized, double-blinded, placebo-controlled, Phase 3 trials, TMC125-C206 and TMC125-C216 (DUET-1 and DUET-2). These trials are identical in design and the results below are pooled data from the two trials.
TMC125-C206 and TMC125-C216 are Phase 3 studies designed to evaluate the safety and antiretroviral activity of INTELENCE® in combination with a background regimen (BR) as compared to placebo in combination with a BR. Eligible subjects were treatment-experienced HIV-1-infected patients with plasma HIV-1 RNA greater than 5000 copies per mL while on an antiretroviral regimen for at least 8 weeks. In addition, subjects had 1 or more NNRTI resistance-associated mutations at screening or from prior genotypic analysis, and 3 or more of the following primary PI mutations at screening: D30N, V32I, L33F, M46I/L, I47A/V, G48V, I50L/V, V82A/F/L/S/T, I84V, N88S, or L90M. Randomization was stratified by the intended use of enfuvirtide (ENF) in the BR, previous use of darunavir/ritonavir (DRV/rtv), and screening viral load. Virologic response was defined as HIV-1 RNA less than 50 copies per mL at Week 48.
All study subjects received DRV/rtv as part of their BR, and at least 2 other investigator-selected antiretroviral drugs (N[t]RTIs with or without ENF). Of INTELENCE®-treated subjects, 25.5% used ENF for the first time (de novo) and 20.0% re-used ENF. Of placebo-treated subjects, 26.5% used de novo ENF and 20.4% re-used ENF.
In the pooled analysis for TMC125-C206 and TMC125-C216, demographics and baseline characteristics were balanced between the INTELENCE® arm and the placebo arm. Table 10 displays selected demographic and baseline disease characteristics of the subjects in the INTELENCE® and placebo arms.
| Pooled TMC125-C206 and TMC125-C216 Trials | ||
|---|---|---|
| INTELENCE® + BR N=599 | Placebo + BR N=604 |
|
| RAMs = Resistance-Associated Mutations, BR=background regimen FC = fold change in EC50 |
||
|
||
| Demographic Characteristics | ||
| Median Age, years (range) | 46 (18–77) | 45 (18–72) |
| Sex | ||
| Male | 90.0% | 88.6% |
| Female | 10.0% | 11.4% |
| Race | ||
| White | 70.1% | 69.8% |
| Black | 13.2% | 13.0% |
| Hispanic | 11.3% | 12.2% |
| Asian | 1.3% | 0.6% |
| Other | 4.1% | 4.5% |
| Baseline Disease Characteristics | ||
| Median Baseline Plasma HIV-1 RNA (range), log10 copies/mL | 4.8 (2.7–6.8) | 4.8 (2.2–6.5) |
| Percentage of Subjects with Baseline Viral Load: | ||
| < 30,000 copies/mL | 27.5% | 28.8% |
| ≥ 30,000 copies/mL and | ||
| < 100,000 copies/mL | 34.4% | 35.3% |
| ≥ 100,000 copies/mL | 38.1% | 35.9% |
| Median Baseline CD4+ Cell Count (range), cells/mm3 | 99 (1–789) | 109 (0–912) |
| Percentage of Subjects with Baseline CD4+ Cell Count: | ||
| < 50 cells/mm3 | 35.6% | 34.7% |
| ≥ 50 cells/mm3 and < 200 cells/mm3 | 34.8% | 34.5% |
| ≥ 200 cells/mm3 | 29.6% | 30.8% |
| Median (range) Number of Primary PI Mutations* | 4 (0–7) | 4 (0–8) |
| Percentage of Subjects with Previous Use of NNRTIs: | ||
| 0 | 8.2% | 7.9% |
| 1 | 46.9% | 46.7% |
| >1 | 44.9% | 45.4% |
| Percentage of Subjects with Previous Use of the following NNRTIs: | ||
| Efavirenz | 70.3% | 72.5% |
| Nevirapine | 57.1% | 58.6% |
| Delavirdine | 13.7% | 12.6% |
| Median (range) Number of NNRTI RAMs† | 2 (0–8) | 2 (0–7) |
| Median Fold Change of the Virus for the Following NNRTIs: | ||
| Delavirdine | 27.3 | 26.1 |
| Efavirenz | 63.9 | 45.4 |
| Etravirine | 1.6 | 1.5 |
| Nevirapine | 74.3 | 74.0 |
| Percentage of Subjects with Previous Use of a Fusion Inhibitor | 39.6% | 42.2% |
| Percentage of Subjects with a Phenotypic Sensitivity Score (PSS) for the background therapy ‡ of: | ||
| 0 | 17.0% | 16.2% |
| 1 | 36.5% | 38.7% |
| 2 | 26.9% | 27.8% |
| ≥ 3 | 19.7% | 17.3% |
Efficacy at Week 48 for subjects in the INTELENCE® and placebo arms for the pooled TMC125-C206 and TMC125-C216 study populations are shown in Table 11.
| Pooled TMC125-C206 and TMC125-C216 Trials | ||
|---|---|---|
| INTELENCE® + BR N=599 | Placebo + BR N=604 |
|
| BR=background regimen | ||
| Virologic Responders at Week 48 Viral Load < 50 HIV-1 RNA copies/mL | 359 (60%) | 232 (38%) |
| Virologic Failures (VF) at Week 48 Viral Load ≥ 50 HIV-1 RNA copies/mL | 123 (21%) | 201 (33%) |
| Death | 11 (2%) | 19 (3%) |
| Discontinuations before Week 48: | ||
| due to VF | 58 (10%) | 110 (18%) |
| due to Adverse Events | 31 (5%) | 14 (2%) |
| due to other reasons | 17 (3%) | 28 (5%) |
At Week 48, 70.8% of INTELENCE®-treated subjects achieved HIV-1 RNA less than 400 copies per mL as compared to 46.4% of placebo-treated subjects. The mean decrease in plasma HIV-1 RNA from baseline to Week 48 was -2.23 log10 copies per mL for INTELENCE®-treated subjects and -1.46 log10 copies per mL for placebo-treated subjects. The mean CD4+ cell count increase from baseline for INTELENCE®-treated subjects was 96 cells per mm3 and 68 cells per mm3 for placebo-treated subjects.
Of the study population who either re-used or did not use ENF, 57.4% of INTELENCE®-treated subjects and 31.7% of placebo-treated subjects achieved HIV-1 RNA less than 50 copies per mL. Of the study population using ENF de novo, 67.3% of INTELENCE®-treated subjects and 57.2% of placebo-treated subjects achieved HIV-1 RNA less than 50 copies per mL.
Treatment-emergent CDC category C events occurred in 4% of INTELENCE®-treated subjects and 8.4% of placebo-treated subjects.
Study TMC125-C227 was a randomized, exploratory, active-controlled, open-label, Phase 2b trial. Eligible subjects were treatment-experienced, PI-naïve HIV-1-infected patients with genotypic evidence of NNRTI resistance at screening or from prior genotypic analysis. The virologic response was evaluated in 116 subjects who were randomized to INTELENCE® (59 subjects) or an investigator-selected PI (57 subjects), each given with 2 investigator-selected N(t)RTIs. INTELENCE®-treated subjects had lower antiviral responses associated with reduced susceptibility to the N(t)RTIs and to INTELENCE® as compared to the control PI-treated subjects.
14.2 Treatment-Experienced Pediatric Subjects (6 years to less than 18 years of age)
TMC125-C213, a single-arm, Phase 2 trial evaluating the pharmacokinetics, safety, tolerability, and efficacy of INTELENCE® enrolled 101 antiretroviral treatment-experienced HIV-1 infected pediatric subjects 6 years to less than 18 years of age and weighing at least 16 kg. Subjects eligible for this trial were on an antiretroviral regimen with confirmed plasma HIV-1 RNA of at least 500 copies per mL and viral susceptibility to INTELENCE® at screening.
The median baseline plasma HIV-1 RNA was 3.9 log10 copies per mL, and the median baseline CD4 cell count was 385 × 106 cells per mm3.
At Week 24, 52% of all pediatric subjects had HIV-1 RNA less than 50 copies per mL. The proportion of pediatric subjects with HIV-1 RNA less than 400 copies per mL was 67%. The mean CD4 cell count increase from baseline was 112 × 106 cells per mm3.
16 HOW SUPPLIED/STORAGE AND HANDLING
INTELENCE® 100 mg tablets are supplied as white to off-white, oval tablets containing 100 mg of etravirine. Each tablet is debossed with "TMC125" on one side and "100" on the other side.
| Bottles of 120 | NDC 54868-5864-0 |
INTELENCE® 200 mg tablets are supplied as white to off-white, biconvex, oblong tablets containing 200 mg of etravirine. Each tablet is debossed with "T200" on one side.
| Bottles of 60 | NDC 54868-6368-0 |
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information).
A statement to patients and healthcare providers is included on the product's bottle label: ALERT: Find out about medicines that should NOT be taken with INTELENCE® from your healthcare provider. A Patient Package Insert for INTELENCE® is available for patient information.
Patients should be informed that INTELENCE® is not a cure for HIV infection and that they may continue to develop opportunistic infections and other complications associated with HIV disease. Patients should be told that sustained decreases in plasma HIV RNA have been associated with a reduced risk of progression to AIDS and death. Patients should remain under the care of a physician while using INTELENCE®.
Patients should be advised to avoid doing things that can spread HIV-1 infection to others. Patients should be advised to practice safe sex and to use latex or polyurethane condoms to lower the chance of sexual contact with any body fluids such as semen, vaginal secretions or blood. Patients should also be advised to never re-use or share needles or other injection equipment, or share personal items that can have blood or body fluids on them, such as toothbrushes and razor blades.
Patients should be advised to take INTELENCE® following a meal twice a day as prescribed. The type of food does not affect the exposure to etravirine.
Patients should be instructed to swallow the INTELENCE® tablet(s) whole with a liquid such as water. Patients should be instructed not to chew the tablets. Patients who are unable to swallow the INTELENCE® tablet(s) whole may disperse the tablet(s) in a glass of water. The patient should be instructed to do the following:
- place the tablet(s) in 5 mL (1 teaspoon) of water, or at least enough liquid to cover the medication,
- stir well until the water looks milky,
- if desired, add more water or alternatively orange juice or milk (patients should not place the tablets in orange juice or milk without first adding water). The use of grapefruit juice or warm (greater than 104°F; greater than 40°C) or carbonated beverages should be avoided.
- drink it immediately,
- rinse the glass several times with water, orange juice, or milk and completely swallow the rinse each time to make sure the patient takes the entire dose.
INTELENCE® must always be used in combination with other antiretroviral drugs. Patients should not alter the dose of INTELENCE® or discontinue therapy with INTELENCE® without consulting their physician.
If the patient misses a dose of INTELENCE® within 6 hours of the time it is usually taken, the patient should take INTELENCE® following a meal as soon as possible, and then take the next dose of INTELENCE® at the regularly scheduled time. If a patient misses a dose of INTELENCE® by more than 6 hours of the time it is usually taken, the patient should not take the missed dose and simply resume the usual dosing schedule. Inform the patient that he or she should not take more or less than the prescribed dose of INTELENCE® at any one time.
INTELENCE® may interact with many drugs; therefore, patients should be advised to report to their healthcare provider the use of any other prescription or nonprescription medication or herbal products, including St. John's wort.
Patients should be informed that severe and potentially life-threatening rash has been reported with INTELENCE®. Rash has been reported most commonly in the first 6 weeks of therapy. Patients should be advised to immediately contact their healthcare provider if they develop rash. Instruct patients to immediately stop taking INTELENCE® and seek medical attention if they develop a rash associated with any of the following symptoms as it may be a sign of a more serious reaction such as Stevens-Johnson syndrome, toxic epidermal necrolysis or severe hypersensitivity: fever, generally ill feeling, extreme tiredness, muscle or joint aches, blisters, oral lesions, eye inflammation, facial swelling, swelling of the eyes, lips, mouth, breathing difficulty, and/or signs and symptoms of liver problems (e.g., yellowing of your skin or whites of your eyes, dark or tea colored urine, pale colored stools/bowel movements, nausea, vomiting, loss of appetite, or pain, aching or sensitivity on your right side below your ribs). Patients should understand that if severe rash occurs, they will be closely monitored, laboratory tests will be ordered and appropriate therapy will be initiated.
Patients should be informed that redistribution or accumulation of body fat may occur in patients receiving antiretroviral therapy, including INTELENCE®, and that the cause and long-term health effects of these conditions are not known at this time.
Product of Belgium
Finished Product Manufactured by:
Janssen Cilag S.p.A., Latina, Italy
Manufactured for:
Janssen Therapeutics, Division of Janssen Products, LP, Titusville NJ 08560
© Janssen Pharmaceuticals, Inc. 2008
Distributed by:
Physicians Total Care, Inc.
Tulsa, Oklahoma 74146
Patient Information
INTELENCE® (in-tel-ence)
(etravirine) tablets
Important: Ask your doctor or pharmacist about medicines that should not be taken with INTELENCE®. For more information, see the section "What should I tell my doctor before taking INTELENCE®?".
Read this Patient Information before you start taking INTELENCE® and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your medical condition or your treatment. You and your doctor should discuss your treatment with INTELENCE® when you start taking it and at regular checkups. You should not change or stop treatment without first talking with your doctor.
What is INTELENCE®?
-
INTELENCE® is a prescription HIV medicine that:
- is used with other HIV medicines to treat HIV (Human Immunodeficiency Virus) infection in adults and children 6 years of age and older. HIV is the virus that causes AIDS (Acquired Immune Deficiency Syndrome). INTELENCE® is a type of HIV medicine called a non-nucleoside reverse transcriptase inhibitor (NNRTI).
- is used in people who are already taking or have taken NNRTI and other HIV medicines and these medicines are not controlling their HIV infection.
- It is not known if INTELENCE® is safe and effective in children less than 6 years of age.
When used with other HIV medicines, INTELENCE® may:
- Reduce the amount of HIV in your blood (called "viral load").
- Help increase the number of CD4 (T) cells in your blood which help fight off other infections.
Reducing the amount of HIV and increasing the CD4 (T) cell count may improve your immune system. This may reduce your risk of death or infections that can happen when your immune system is weak (opportunistic infections).
INTELENCE® does not cure HIV infection or AIDS.
You must stay on continuous HIV therapy to control HIV infection and decrease HIV-related illnesses.
Avoid doing things that can spread HIV-1 infection to others:
- Do not share or re-use needles or other injection equipment.
- Do not share personal items that can have blood or body fluids on them, like toothbrushes and razor blades.
- Do not have any kind of sex without protection. Always practice safe sex by using a latex or polyurethane condom to lower the chance of sexual contact with semen, vaginal secretions, or blood.
Ask your doctor if you have any questions on how to prevent passing HIV to other people.
What should I tell my doctor before taking INTELENCE®?
Before taking INTELENCE® tell your doctor if you:
- have had or currently have liver problems, including hepatitis B or C.
- have any other medical conditions.
- are pregnant or plan to become pregnant. It is not known if INTELENCE® will harm your unborn baby.
Pregnancy Registry: There is a pregnancy registry for women who take antiviral medicines during pregnancy. The purpose of this registry is to collect information about the health of you and your baby. Talk to your doctor about how you can take part in this registry. - are breastfeeding or plan to breastfeed. Do not breastfeed if you take INTELENCE®. You should not breastfeed if you have HIV because of the risk of passing HIV to your baby. Talk with your doctor about the best way to feed your baby.
Tell your doctor about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements, including St. John's wort (Hypericum perforatum). Some medicines may interact with INTELENCE®.
INTELENCE® may affect the way other medicines work, and other medicines may affect how INTELENCE® works. Taking INTELENCE® and certain other medicines may cause serious side effects. If you take certain medicines with INTELENCE®, then the amount of INTELENCE® in your body may be too low and INTELENCE® may not work to help reduce your HIV viral load. The HIV virus in your body may become resistant to INTELENCE® or other HIV medicines that are like it.
Tell your doctor if you take other HIV medicines. Some HIV medicines should not be taken with INTELENCE®.
Tell your doctor if you take:
- an antiarrhythmic to treat abnormal heart rhythms-amiodarone (Cordarone®, Pacerone®), bepridil (Vascor®), digoxin (Lanoxin®), disopyramide (Norpace®), flecainide (Tambocor™), lidocaine (LidoPen®, Xylocaine®), mexiletine (Mexitil®), propafenone (Rythmol SR®), quinidine (Duraquin®, Quinaglute®)
- an anticoagulant to prevent blood clots-warfarin (Coumadin®)
- an anti-seizure medicine to treat epilepsy or prevent seizures-carbamazepine (Tegretol®, Tegretol-XR®, Carbatrol®, Equetro®, Teril®, Epitol®), phenobarbital (Luminal®, Solfoton®), phenytoin (Dilantin®, Dilantin-125®, Phenytek®)
- an antifungal to treat a fungal infection-fluconazole (Diflucan®), itraconazole (Onmel®, Sporanox®), ketoconazole (Nizoral®), posaconazole (Noxafil®), voriconazole (Vfend®)
- an anti-infective to treat a bacterial infection-clarithromycin (Biaxin®, Biaxin XL®)
- an antimycobacterial to treat a bacterial infection, including tuberculosis (TB)-rifabutin (Mycobutin®), rifampin (Rifadin®, Rifater®, Rifamate®, Rimactane®), rifapentine (Priftin®)
- a benzodiazepine to treat trouble with sleeping and/or anxiety-diazepam (Diastat®, Valium®)
- a corticosteroid to treat inflammation or asthma-dexamethasone (Decadron®)
- an HMG-CoA reductase inhibitor to lower cholesterol levels-atorvastatin (Caduet®, Lipitor®), fluvastatin (Lescol®), lovastatin (Advicor®, Altoprev®, Mevacor®), pitavastatin (Livalo®), rosuvastatin (Crestor®), simvastatin (Juvisync®, Simcor®, Vytorin®, Zocor®)
- an immunosuppressant to prevent organ transplant rejection-cyclosporine (Gengraf®, Sandimmune®, Neoral®), sirolimus (Rapamune®), tacrolimus (Prograf®)
- a medicine to treat narcotic withdrawal and dependence-buprenorphine (Buprenex®, Butrans®, Subutex®), buprenorphine/naloxone (Suboxone®), methadone (Dolophine®, Methadose®)
- a PDE-5 inhibitor to treat erectile dysfunction and/or pulmonary arterial hypertension-sildenafil (Revatio®, Viagra®), tadalafil (Adcirca®, Cialis®), vardenafil (Levitra®, Staxyn™)
- a platelet aggregation inhibitor to prevent blood clots-clopidogrel (Plavix®)
Ask your doctor or pharmacist if you are not sure if your medicine is one that is listed above.
Know the medicines you take. Keep a list of your medicines and show it to your doctor and pharmacist when you get a new medicine.
Your doctor and your pharmacist can tell you if you can take these medicines with INTELENCE®. Do not start any new medicines while you are taking INTELENCE® without first talking with your doctor or pharmacist. You can ask your doctor or pharmacist for a list of medicines that can interact with INTELENCE®.
How should I take INTELENCE®?
- Stay under the care of your doctor during treatment with INTELENCE®.
- Take INTELENCE® tablets every day exactly as prescribed by your doctor.
- Your doctor will tell you how many INTELENCE® tablets to take and when to take them. Talk to your doctor if you have questions about when to take INTELENCE®.
- INTELENCE® is taken two times each day.
- -
- If your child takes INTELENCE®, your doctor will prescribe the right dose based on your child's weight.
- Always take INTELENCE® following a meal. Do not take INTELENCE® on an empty stomach. INTELENCE® may not work as well if you take it on an empty stomach.
- Do not change your dose or stop taking INTELENCE® without first talking with your doctor. See your doctor regularly while taking INTELENCE®.
- Swallow INTELENCE® whole, with a glass of water. Do not chew the tablet(s).
- If you are unable to swallow INTELENCE® tablets whole, you may take your dose of INTELENCE® as follows:
- Step 1: Measure approximately 5 mL (1 teaspoon) of water and pour into a cup.
- Step 2: Place the tablets in the cup containing 5 mL of water. If needed, add more water to cover the tablets. Do not put the tablets in other liquids.
- Step 3: Stir well until the water looks milky.
- Step 4: At this step, you may add a small amount of water, orange juice or milk to make it easier to take.
- Step 5: Drink right away.
- Step 6: Add more water, orange juice or milk to the cup to rinse the cup several times and completely swallow each time to make sure you take your entire dose of INTELENCE®.
- Avoid using grapefruit juice or warm (more than 104°F or 40°C) or carbonated beverages when taking INTELENCE® tablets.
- When your supply of INTELENCE® starts to run low, get more from your doctor or pharmacy. It is important not to run out of INTELENCE®. The amount of HIV in your blood may increase if the medicine is stopped even for a short time.
- If you miss a dose of INTELENCE® within 6 hours of the time you usually take it, take your dose of INTELENCE® following a meal as soon as possible. Then, take your next dose of INTELENCE® at the regularly scheduled time. If you miss a dose of INTELENCE® by more than 6 hours of the time you usually take it, wait and then take the next dose of INTELENCE® at the regularly scheduled time.
- Do not take more than your prescribed dose to make up for a missed dose.
- If you take too much INTELENCE®, contact your local poison control center or go to the nearest emergency room right away.
What are the possible side effects of INTELENCE®?
INTELENCE® can cause serious side effects including:
-
Severe skin rash and allergic reactions. Skin rash is a common side effect of INTELENCE®. Rash can be serious. Call your doctor right away if you get a rash. In some cases, severe rash and allergic reaction may need to be treated in a hospital and may potentially lead to death.
If you get a rash with any of the following symptoms, stop taking INTELENCE® and call your doctor or get medical help right away:- hives or sores in your mouth, or your skin blisters and peels
- trouble swallowing or breathing
- swelling of your face, eyes, lips, tongue, or throat
- fever, yellowing of the skin or whites of the eyes, dark urine, or pain on the right side of the stomach-area (abdominal pain).
- Changes in body fat can happen in people taking HIV medicines. These changes may include an increased amount of fat in the upper back and neck ("buffalo hump"), breast, and around the middle of your body (trunk). Loss of fat from the legs, arms, and face may also happen. The exact cause and long-term health effects of these problems are not known.
- Changes in your immune system (Immune Reconstitution Syndrome) can happen when you start taking HIV medicines. Your immune system may get stronger and begin to fight infections that have been hidden in your body for a long time. Call your doctor right away if you start having any new symptoms after starting your HIV medicine.
In adults, common side effects of INTELENCE® include tingling, numbness, or pain in the hands or feet.
In children, diarrhea is a common side effect of INTELENCE®.
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects with INTELENCE®. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store INTELENCE®?
- Store INTELENCE® tablets at 59°F to 86°F (15°C to 30°C).
- Keep INTELENCE® in the original bottle given to you by your pharmacist. After opening, keep the bottle tightly closed to protect INTELENCE® from moisture.
- The INTELENCE® bottle contains a desiccant packet to help keep your medicine dry (protect it from moisture). The bottle of 25 mg tablets contains 2 desiccant packets. The bottles of 100 mg and 200 mg tablets contain 3 desiccant packets. Keep the desiccant packets in the bottle. Do not eat the desiccant packets.
Keep INTELENCE® and all medicines out of the reach of children.
General information about INTELENCE®
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use INTELENCE® for a condition for which it was not prescribed. Do not give INTELENCE® to other people even if they have the same condition you have. It may harm them.
This leaflet summarizes the most important information about INTELENCE®. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about INTELENCE® that is written for health professionals. For more information, you may also call Janssen Products, LP at 1-800-526-7736.
What are the ingredients in INTELENCE®?
Active ingredient: etravirine.
25 mg and 100 mg INTELENCE® tablets contain the following inactive ingredients: hypromellose, microcrystalline cellulose, colloidal silicon dioxide, croscarmellose sodium, magnesium stearate and lactose monohydrate
200 mg INTELENCE® tablets contain the following inactive ingredients: hypromellose, silicified microcrystalline cellulose, microcrystalline cellulose, colloidal silicon dioxide, croscarmellose sodium and magnesium stearate.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Product of Belgium
Finished Product Manufactured by:
Janssen Cilag S.p.A., Latina, IT
Manufactured for:
Janssen Therapeutics, Division of Janssen Products, LP, Titusville NJ 08560
INTELENCE® is the registered trademark of Tibotec Pharmaceuticals.
Revised August 2012
© Janssen Pharmaceuticals, Inc. 2008

