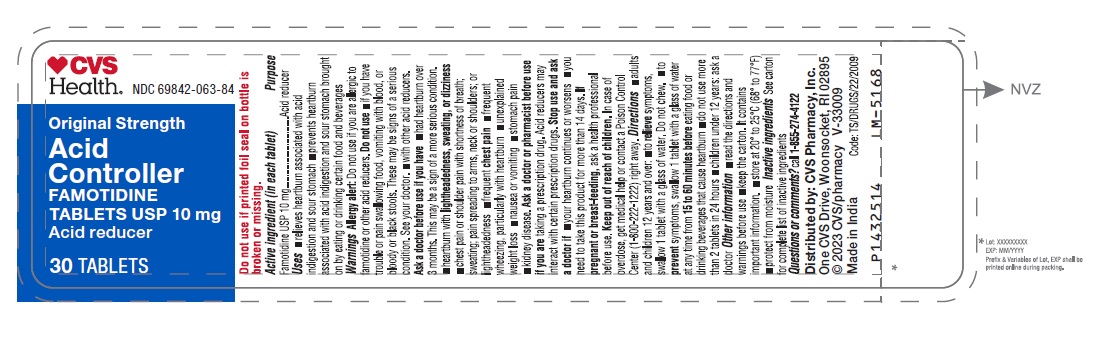
Uses
- relieves heartburn associated with acid indigestion and sour stomach
- prevents heartburn associated with acid indigestion and sour stomach brought on by eating or drinking certain food and beverages
Do not use
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools.These may be signs of a serious condition. See your doctor.
- with other acid reducers
Ask a doctor before use if you have
- had heartburn over 3 months. This may be a sign of a more serious condition.
- heartburn with lightheadedness, sweating, or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- frequent chest pain
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
- kidney disease
Ask a doctor or pharmacist before use if you are
taking a prescription drug. Acid reducers may interact with certain prescription drugs.
Stop use and ask a doctor if
- your heartburn continues or worsens
- you need to take this product for more than 14 days
Keep out of reach of children
In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
- adults and children 12 years and over:
- to relieve symptoms, swallow 1 tablet with a glass of water. Do not chew.
- to prevent symptoms, swallow 1 tablet with a glass of water at any time from 15 to 60 minutes before eating food or drinking beverages that cause heartburn
- do not use more than 2 tablets in 24 hours
- children under 12 years: ask a doctor
Other information
- read the directions and warnings before use
- keep the carton. It contains important information.
- store at 20° to 25°C (68° to 77°F)
- protect from moisture
Inactive ingredients
carnauba wax, corn starch, hydroxypropyl cellulose, hypromellose, magnesium stearate, microcrystalline cellulose, red iron oxide, sodium starch glycolate, talc and titanium dioxide.
Questions or comments?
call 1-855-274-4122
Tips for Managing Heartburn
- Do not lie flat or bend over after eating
- Do not wear tight-fitting clothing around the stomach
- Do not eat before bedtime
- Raise the head of your bed
- Avoid heartburn-causing foods such as rich, spicy, fatty or fried foods, chocolate, caffeine, alcohol, and certain fruits and vegetables
- Eat slowly and avoid big meals
- If overweight, lose weight
- Quit smoking
JUST ONE TABLET prevents and relieves heartburn due to acid indigestion brought on by eating and drinking certain foods and beverages.
Do not use if carton is open or if printed foil seal under bottle cap is open or torn.
Distributed by:
CVS Pharmacy, Inc.
One CVS Drive, Woonsocket, RI 02895
© 2023 CVS/pharmacy
CVS.com®
1-800-SHOP CVS
Made in India V-33009
Code: TS/DRUGS/22/2009
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL -10 mg (30 Tablets Container Label)
♥CVS
Health®
NDC 69842-063-84
Original Strength
Acid Controller
FAMOTIDINE
TABLETS USP 10 mg
Acid reducer
30 TABLETS
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL -10 mg (30 Tablets Container Carton Label)
♥CVS
Health®
Compare to the active ingredient
of Original Strength Pepcid® AC*
NDC 69842-063-84
Original Strength
Acid
Controller
FAMOTIDINE TABLETS
USP 10 mg
Acid reducer
Just one tablet:
Prevents & relieves heartburn
due to acid indigestion
Actual Size
Package Contains
One Bottle
30 TABLETS
