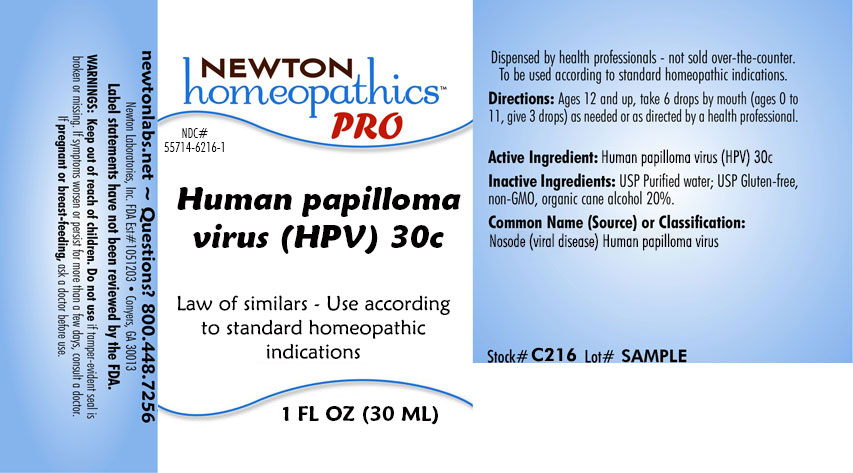
HUMAN PAPILLOMA VIRUS (HPV)- human papilloma virus (hpv) liquid
Newton Laboratories, Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
INDICATIONS & USAGE SECTION
Law of similars - Use according to standard homeopathic indications
DOSAGE & ADMINISTRATION SECTION
Directions: Ages 12 and up, take 6 drops by mouth (ages 0 to 11, give 3 drops) as needed or as directed by a health professional.
ACTIVE INGREDIENT SECTION
Human Papilloma Virus 30c
PURPOSE SECTION
Law of similars - Use according to standard homeopathic indications
INACTIVE INGREDIENT SECTION
USP Purified water; USP Gluten-free, non-GMO, organic cane alcohol 20%.
QUESTIONS SECTION
newtonlabs.net - Questions? 800.448.7256
Newton Laboratories, Inc. FDA Est # 1051203 - Conyers, GA 30013
WARNINGS SECTION
WARNINGS: Keep out of reach of children. Do not use if tamper-evident seal is broken or missing. If symptoms worsen or persist for more than a few day, consult a doctor. If
pregnant or breast-feeding, ask a doctor before use.
OTC - PREGNANCY OR BREAST FEEDING SECTION
If
pregnant or breast-feeding, ask a doctor before use.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children.
PACKAGE LABEL
Newton Laboratories, Inc.
