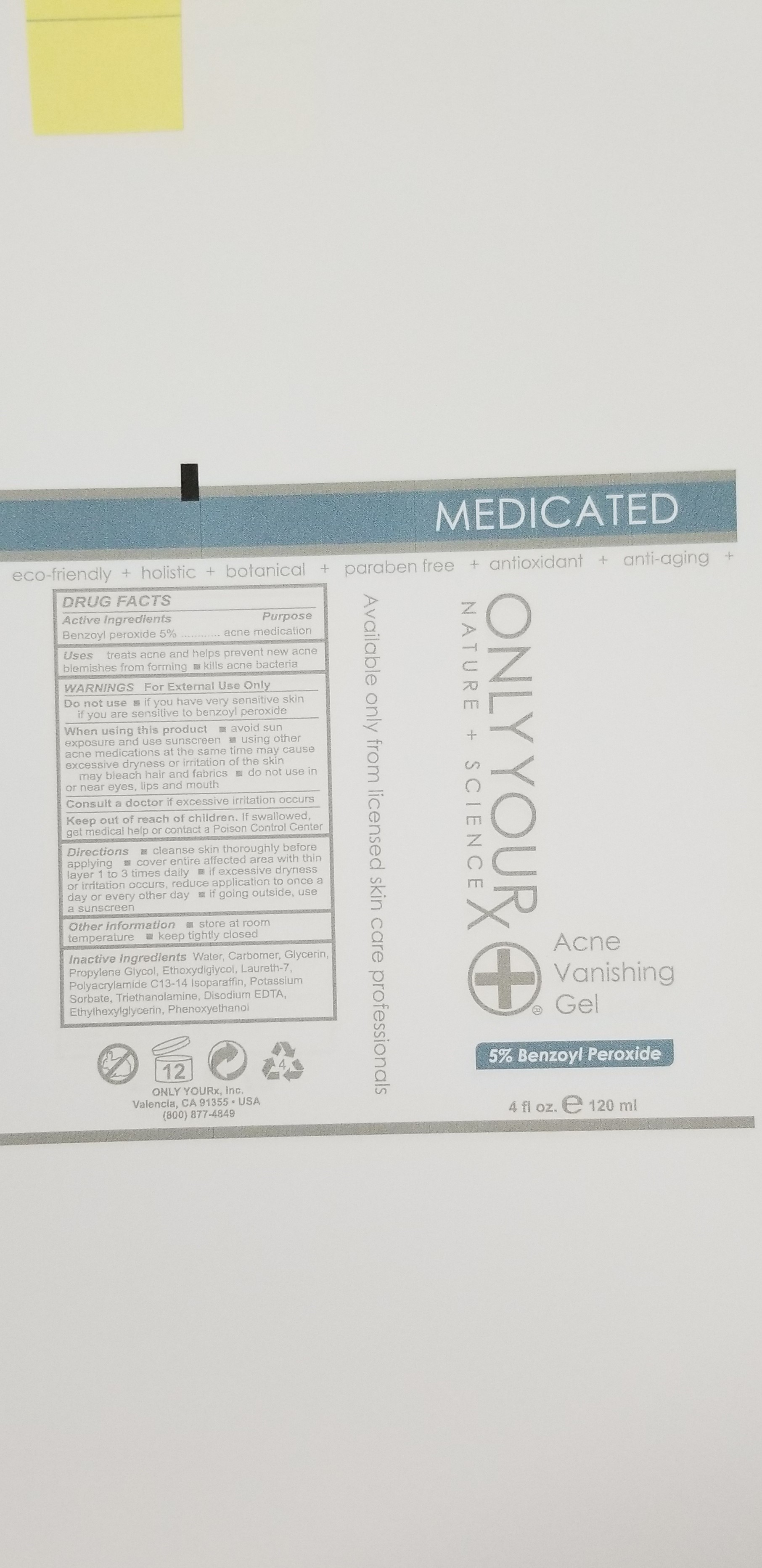
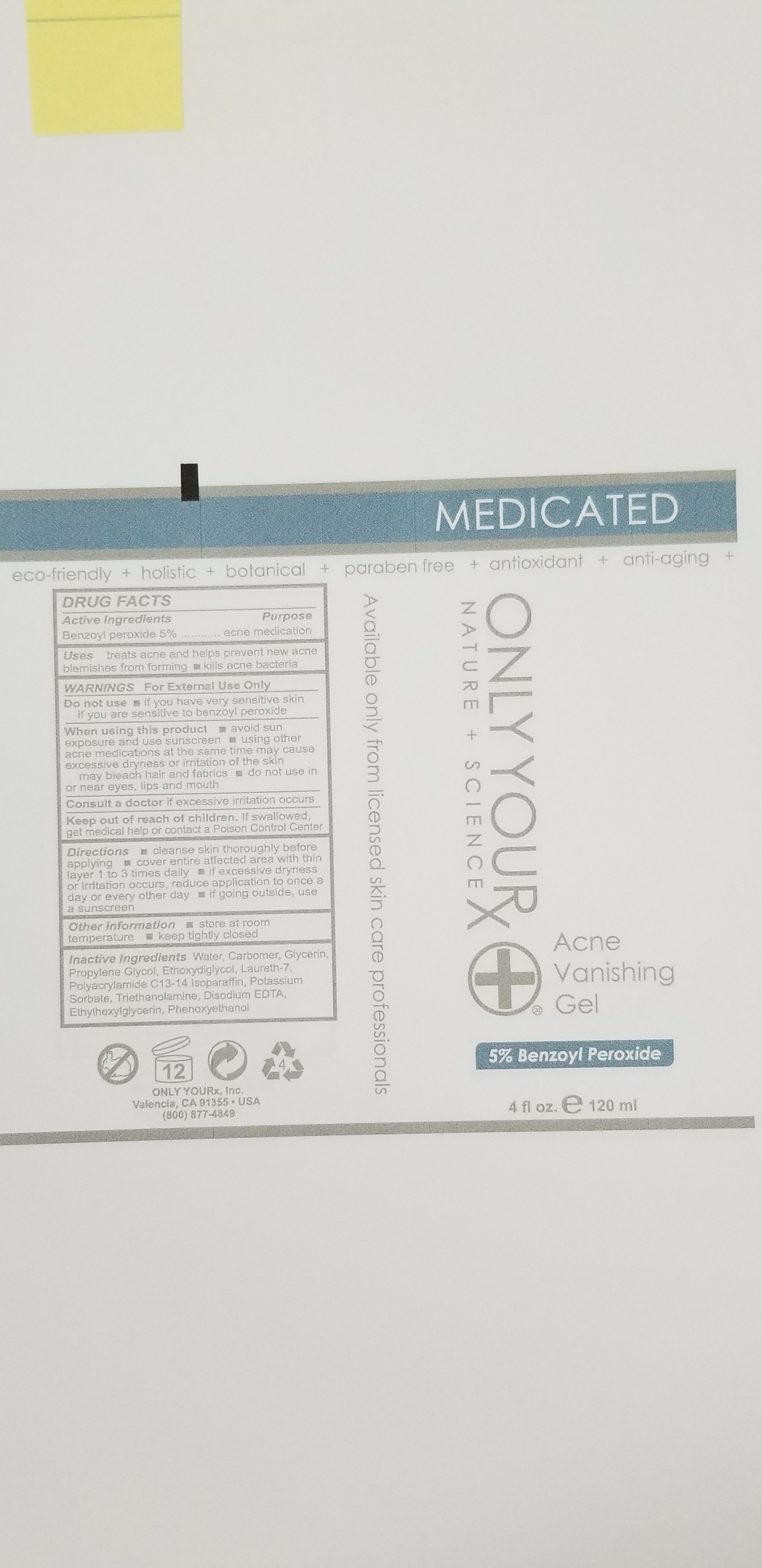
ACNE VANISHING GEL- 5%- benzoyl peroxide gel
Only Yourx, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Water, Carbomer, Glycerin, Propylene Glycol, Ethoxydigiycol, Laureth-7, Polyacrylamide C13-14 Isoparaffin, Potassium Sorbate, Triethanolamine, Disodium EDTA, Ethylhexylglycerin, Phenoxyethanol
treats acne and helps prevent new acne blemishes from forming
kills acne bacteria
Directions:
- Cleanse skin thoroughy before applying
- Cover entire affected area with thin layer 1 to 3 times daily
- If excessive dryness or irritation occurs, reduce application to once a day or every other day
- If going outside, use a sunscreen
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center
WARNINGS: For External Use Only
Other Information
- Store at room temperature
- Keep tightly closed
Do not use
- If you have very sensitive skin
- If you are sensitive to benzoyl peroxide
When using this product:
- avoid sun exposure and use sunscreen
- using other acne medications at the same time may cause excessive dryness or irritation of the skin
- may bleach hair and fabrics
- do not use in or near eyes, lips and mouth
Consult a doctor if excessive irritation occurs
Only Yourx, Inc.