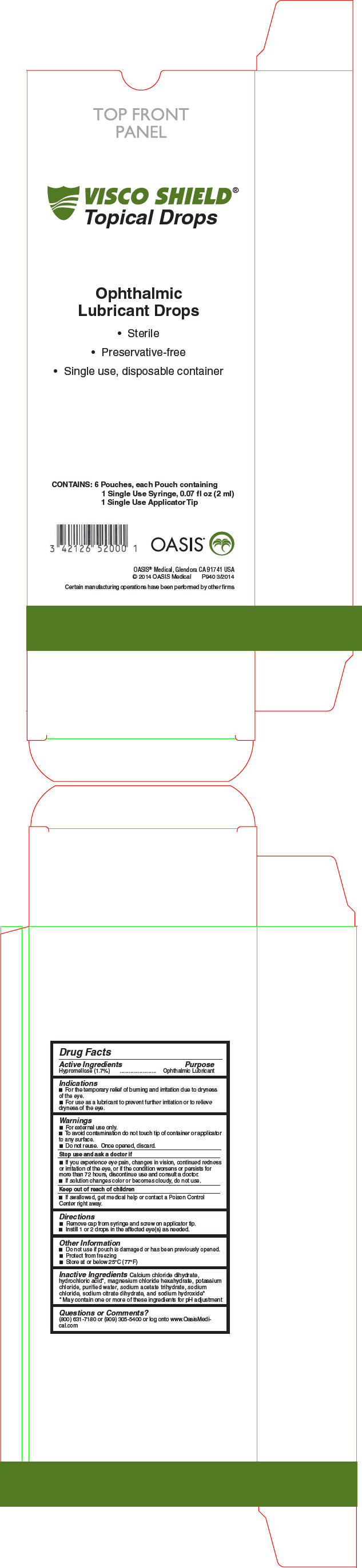
Uses
- For the temporary relief of burning and irritation due to dryness of the eye
- For use as a lubricant to prevent further irritation or to relieve dryness of the eye
Warnings
- For external use only
- To avoid contamination do not touch tip of container or applicator to any surface
- Once opened, discard
- Do not reuse
Directions
- Remove cap from syringe and screw on applicator tip
- Instill 1 or 2 drops in the affected eye(s) as needed.
Other information
- Do not sue if pouch is damage or has been previously opened
- Protect from freezing
- Store at or below 77°F/25°C
Inactive Ingredients
Calcium chloride dihydrate, hydrochloric acid1, magnesium chloride hexahydrate, potassium chloride, purified water, sodium acetate trihydrate, sodium chloride, sodium citrate dihydrate, and sodium hydroxide1
- 1
- May contain one or more of these ingredients for pH adjustment
PRINCIPAL DISPLAY PANEL - 2 ml Syringe Carton
VISCO SHIELD®
Topical Drops
Ophthalmic
Lubricant Drops
- Sterile
- Preservative-free
- Single use, disposable container
CONTAINS: 6 Pouches, each Pouch containing
1 Single Use Syringe, 0.07 fl oz (2 ml)
1 Single Use Applicator Tip
OASIS®
OASIS® Medical, Glendora CA 91741 USA
© 2014 OASIS Medical
P940 3/2014
Certain manufacturing operations have been performed by other firms