ACTIVE INGREDIENTS PURPOSE
Pyrithione Zinc 0.19%.................. Antidandruff/ Seborrheic Dermatitis
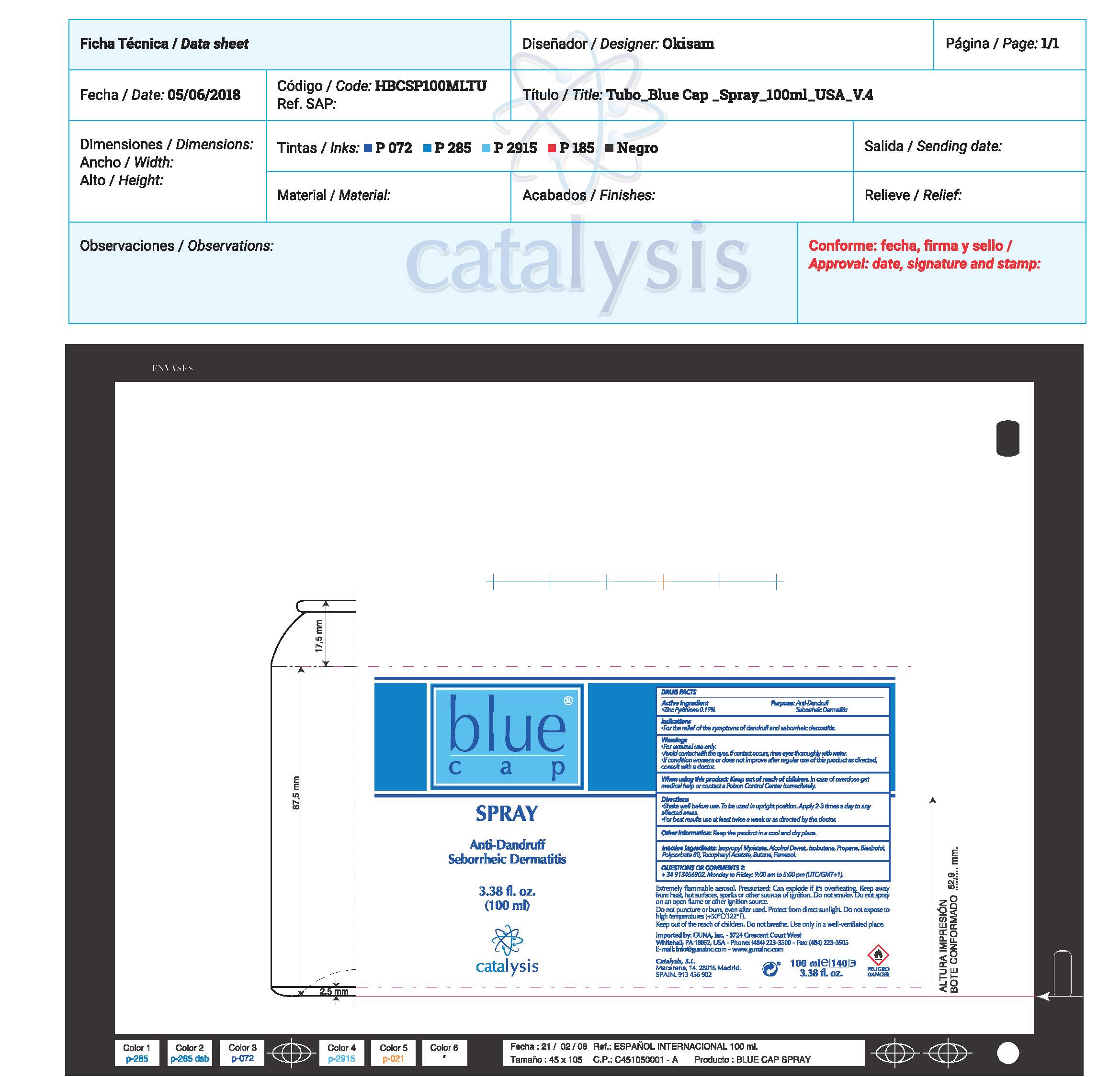
Warnings
- For external use only.
- Avoid contact with the eyes. If contact occurs, rinse throughly with water
- If conditions worsens or does not improve after regular use of this product as directed, consult with a doctor
- When using this product KEEP out of the reach of children. In case of overdose get medical help or contact a Poison Center inmediately
WARNINGS
- For external use only.
- Avoid contact with the eyes. If contact occurs, rinse throughly with water
- If conditions worsens or does not improve after regular use of this product as directed, consult with a doctor
- When using this product KEEP out of the reach of children. In case of overdose get medical help or contact a Poison Center inmediately
Directions
- Shake well before use. To be use in upright position. Apply 2-3 times a day to any affected area.
- For best results use at least twice a week or as directed by the doctor
