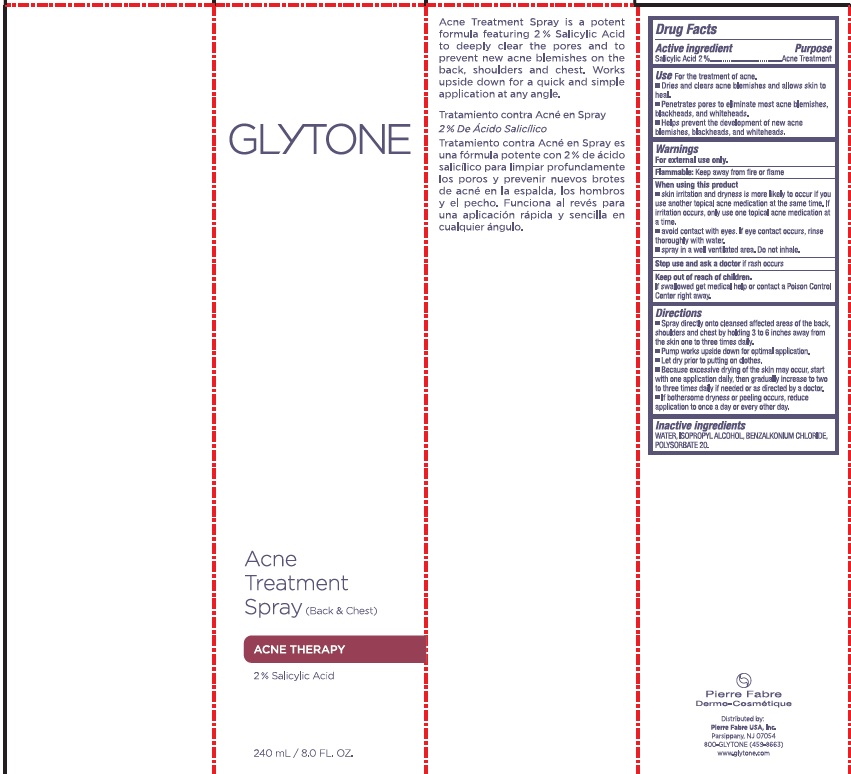
Use
For the treatment of acne.
- Dries and clears acne blemishes and allows skin to heal.
- Penetrates pores to eliminate most acne blemishes, blackheads, and whiteheads.
- Helps prevent the development of new acne blemishes, blackheads, and whiteheads.
Warnings
When using this product
- Skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs use only one topical acne medication at a time.
- Avoid contact with eyes. If eye contact occurs, rinse thoroughly with water.
- Spray in a well ventilated area. Do not inhale.
Directions
Spray directly onto cleansed affected areas of the back, shoulders and chest by holding 3 to 6 inches away from the skin one to three times daily.
Pump works upside down and for optimal application.
Let dry prior to putting on clothes.
Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two to three times daily if needed or as directed by a doctor.
Inactive Ingredients
AQUA (WATER), ALCOHOL DENAT., METHYLPROPANEDIOL, GLUCONOLACTONE, POLYSORBATE 20, POTASSIUM SORBATE, SODIUM GLUCONATE, SODIUM BENZOATE, CITRIC ACID, GLYCERIN, HIBISCUS SARBARIFFA FLOWER EXTRACT, CENTELLA ASIATICA (GOTU KOLA) LEAF EXTRACT, CAMELLIA SINENSIS (WHITE TEA) LEAF EXTRACT, CAMELLIA OLEIFERA (GREEN TEA) LEAF EXTRACT, ALOE BARBADENSIS LEAF JUICE POWDER, SODIUM HYDROXIDE