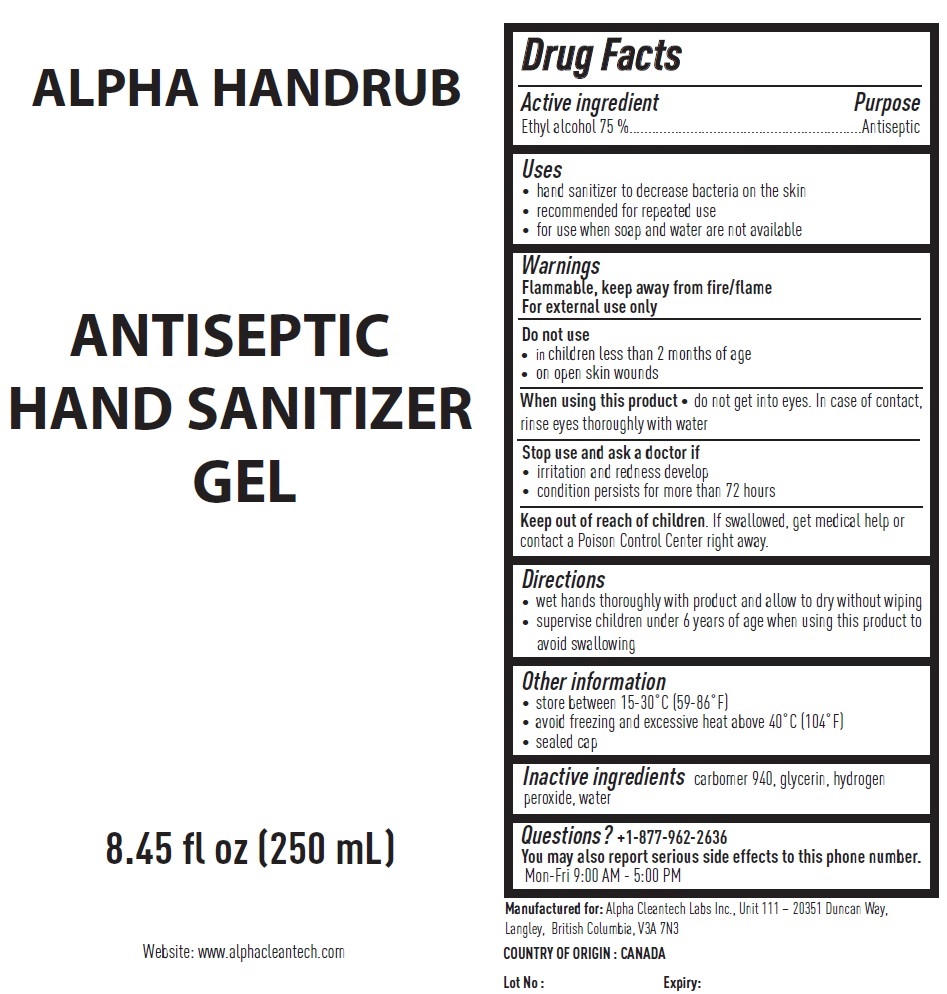
Uses
• hand sanitizer to decrease bacteria on the skin
• recommended for repeated use
• for use when soap and water are not available
Warnings
Flammable, keep away from fire/flame
For external use only
Do not use
• in children less than 2 months of age
• on open skin wounds
When using this product • do not get into eyes. In case of contact, rinse eyes thoroughly with water
Stop use and ask a doctor if
• irritation and redness develop
• condition persists for more than 72 hours
Directions
• wet hands thoroughly with product and allow to dry without wiping
• supervise children under 6 years of age when using this product to avoid swallowing
Other information
• store between 15-30°C (59-86°F)
• avoid freezing and excessive heat above 40°C (104°F)
• sealed cap
Questions?
+1-877-962-2636
You may also report serious side effects to this phone number.
Mon-Fri 9:00 AM - 5:00 PM