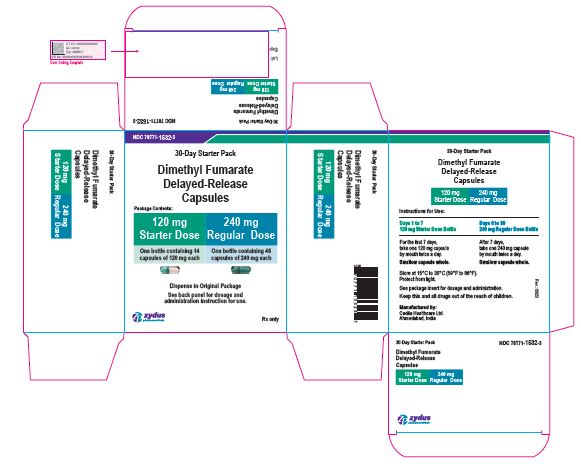
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
30-Day Starter Pack Carton Label
Dimethyl Fumarate Delayed-release Capsules
120 mg Starter Dose: 14 Capsules
240 mg Regular Dose: 46 Capsules
Rx only
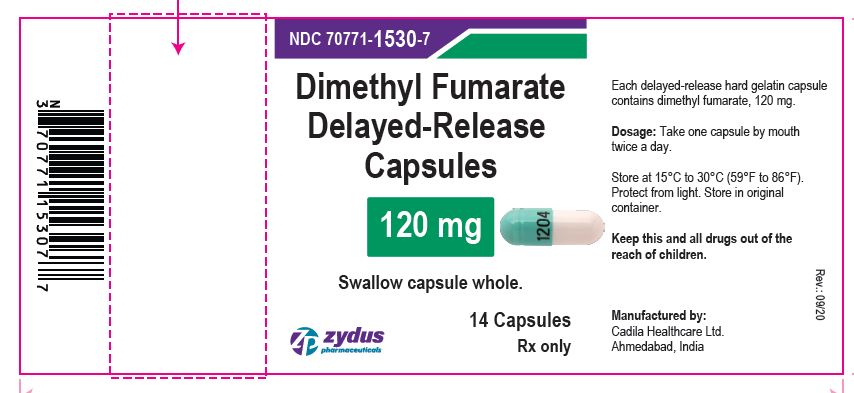
Dimethyl Fumarate Delayed-release Capsules, 120 mg
14 Capsules
Rx only
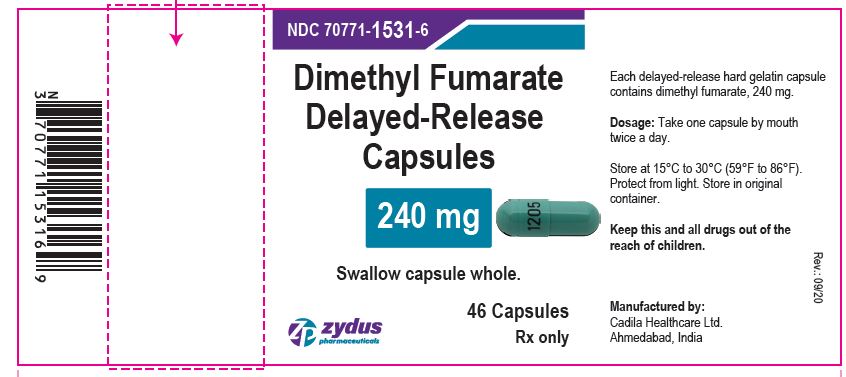
Dimethyl Fumarate Delayed-release Capsules, 240 mg
60 Capsules
Rx only