Drug Facts Active Ingredients
All actives are HPUS* and 3X potency.
Aesculus Hipp., Calendula Off., Equisetum Arv., Symphytum Off.
Salix Alba, Setllaria Med.
Mezereum Off., Urtica Urens
Althaea Off., Hamamelis Vir.
*HPUS indicates the active ingredients are in the official Homeopathic Pharmacopeia of the United States.
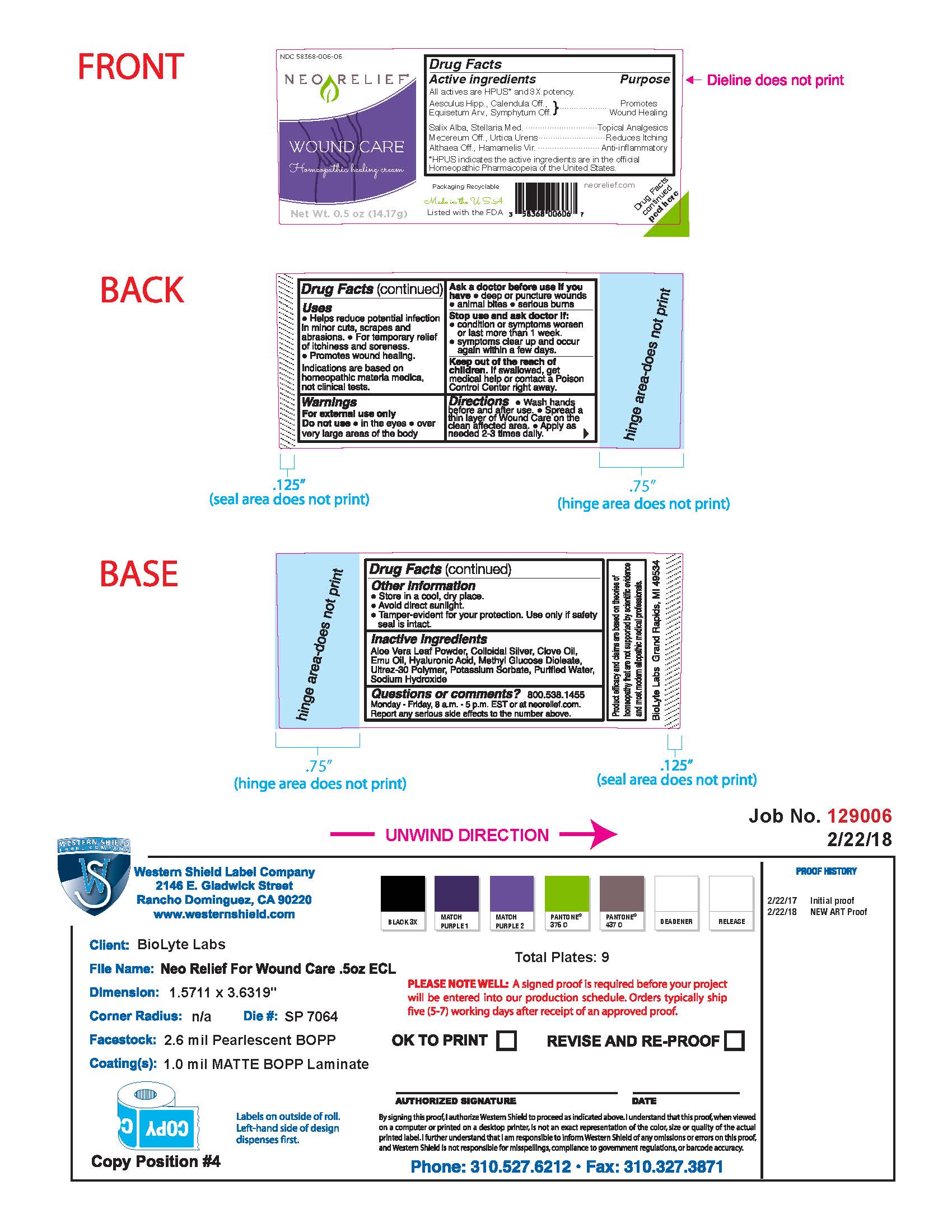
Uses
Helps reduce potential infection in minor cuts, scrapes and abrasions.
For the temporary relief of itchiness and soreness.
Promotoes wound healing.
Indications are based on homeopathic materia medica, not clinical tests.
Warnings
Stop use and ask doctor if condition or symptoms worsen or last more than 1 week; symptoms clear up and occur again within a few days.
Warnings
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Wash hands before and after use; spread a thin layer of Wound Care on the clean affected area; Apply as needed 2-3 times daily.
Other Information
Store in a cool, dry place.
Avoid direct sunlight.
Tamper-evident for your protection. Use only if safety seal is intact.
Inactive Ingredients
Aloe Vera Leaf Powder, Colloidal Silver, Clove Oil, Emu Oil, Hyaluronic Acid, Methyl Glucose Dioleate, Ultrez-30 Polymer, Potassium Sorbate, Purified Water, Sodium Hydroxide
Questions or Comments?
800.538.1455
Monday-Friday, 8 a.m. - 5 p.m. EST or at neorelief.com.
Report any serious side effects to the number above.
Addtional Label Content
Packaging Recyclable
Made in the USA
Listed with the FDA
neorelief.com
Drug Facts peeled here
FTC disclosure: Product efficacy and claims are based on theories of homeopathy that are not supported by scientific evidence and most modern allopathic medical professionals.
BioLyte Labs Grand Rapids, MI 49534