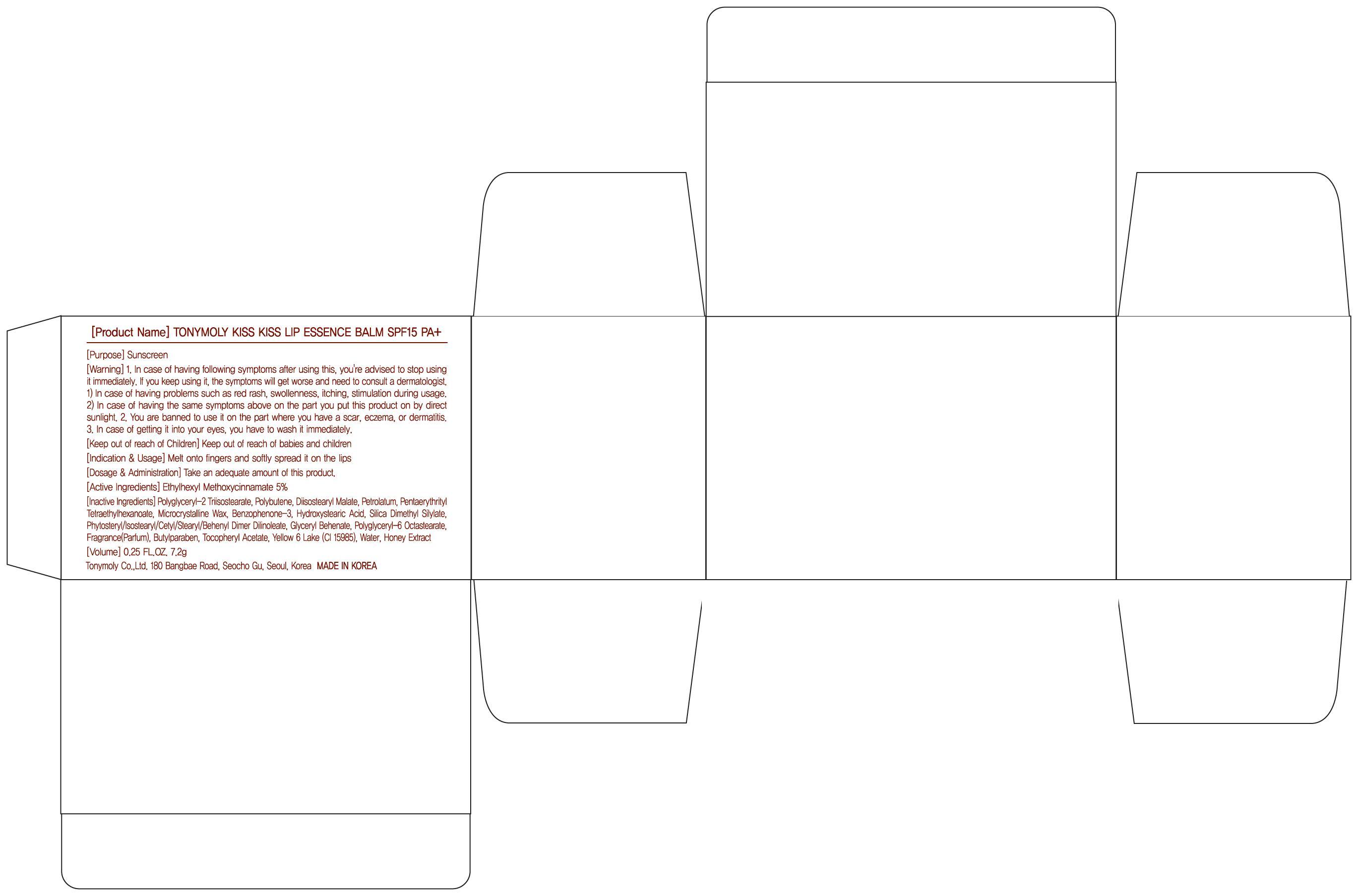
INACTIVE INGREDIENT
Inactive Ingredients:
Polyglyceryl-2 Triisostearate, Polybutene, Diisostearyl Malate, Petrolatum, Pentaerythrityl Tetraethylhexanoate, Microcrystalline Wax, Benzophenone-3, Hydroxystearic Acid, Silica Dimethyl Silylate, Phytosteryl/Isostearyl/Cetyl/Stearyl/Behenyl Dimer Dilinoleate, Glyceryl Behenate, Polyglyceryl-6 Octastearate, Fragrance(Parfum), Butylparaben, Tocopheryl Acetate, Yellow 6 Lake (CI 15985), Water, Honey Extract
WARNINGS
Warning:
1. In case of having following symptoms after using this, you're advised to stop using it immediately. If you keep using it, the symptoms will get worse and need to consult a dermatologist.
1) In case of having problems such as red rash, swollenness, itching, stimulation during usage.
2) In case of having the same symptoms above on the part you put this product on by direct sunlight.
2. You are banned to use it on the part where you have a scar, eczema, or dermatitis.
3. In case of getting it into your eyes, you have to wash it immediately.